Optical-resolution photoacoustic microscopy continually monitors macrophages activities of acute inflammation in vivo  Download: 962次
Download: 962次
Macrophages are a heterogeneous population of resident and recruited cells that are found in all organs[1], which are an important part of the mononuclear phagocyte system to maintain the stability of the immune system[2]. Macrophages also play a critical role in tissue repair and remodeling, as well as in the orchestration of the host’s response to infectious diseases through secretion of cytokines, enzymes, and reactive oxygen species[1]. They can display significant plasticity and change their physiology according to environmental cues to produce different cell populations with different functions[3]. In the process of inflammation, macrophages are activated and involved in the autoregulatory loop of inflammation. Their main role is to produce a variety of cytokines and growth factors for antigen presentation, phagocytosis, and immune regulation[4].
Now, in vivo clinical imaging tools for noninvasive macrophage quantification are expected to predict patients’ clinical outcome, define treatment options, and monitor therapy responses[5]. But, imaging the immune cells still focuses on the preclinical study that is limited by the technique development. Optical imaging offers exceptional opportunities to visualize and quantify multiple dynamic events in living cells with high resolution[6]. Fluorescence imaging as a conventional optical imaging technique has opened the possibility of generating bespoke reagents to image cellular activity in real time[79" target="_self" style="display: inline;">–
As a new non-destructive imaging method, photoacoustic (PA) imaging has been widely used in biomedical research, such as brain disease[12,13]. The scale of PA imaging extends from organelles, cells, tissues, and organs to the whole body[14]. Optical-resolution PA microscopy (OR-PAM) has high spatial resolution and large penetration depth. Now, it has many different forms, for example, the portable OR-PAM was used in stomatology[15]. The blind-deconvolution OR-PAM can provide a lateral resolution ∼2-fold finer than that of conventional OR-PAM[16]. OR-PAM has been applied to different biomedical applications, such as monitoring vascular normalization during anti-angiogenic therapy[17], imaging early-stage nanocarrier-enhanced chemotherapy response in living subjects[18], and assessing the burn healing[19]. It was used to monitor the vessels in inflammation induced by lipopolysaccharide[20]. Micro-electro mechanical system (MEMS)-OR-PAM as a rapid imaging technique was also used to monitor the vessel changes[21]. PA imaging was used to image the immune cells labeled with silica-coated nanorods[22] and the immune cell activities at the macro scale[23]. But, these studies did not involve any immune cell activities at the microscopic level to the best of our knowledge.
In this study, we set up an OR-PAM system and further employed the MEMS technique for fast imaging. Its lateral resolution was characterized to be 6.7 μm, allowing us to image both microvessels and cells noninvasively at a rapid speed. OR-PAM was used to image the macrophages labeled with ink in a petri dish. At first, we injected the labeled macrophages through the tail vein. The modeled mouse ear was established right away by smearing xylene evenly. Then, we observed the changes of blood vessels in 92.5 min by OR-PAM. It can be seen that macrophages gathered together near the vessels at the beginning of inflammation and then infiltrated into tissue fluid to swallow the inflammatory substances. With the ear recovered from inflammation, they left away from this region. From quantitative data, we also found the vessel changes in a modeled ear injected with macrophages returned to before in the end. OR-PAM promises to be an important way to provide accurate position information of immune cells for immunotherapy in vivo.
As shown in Fig.
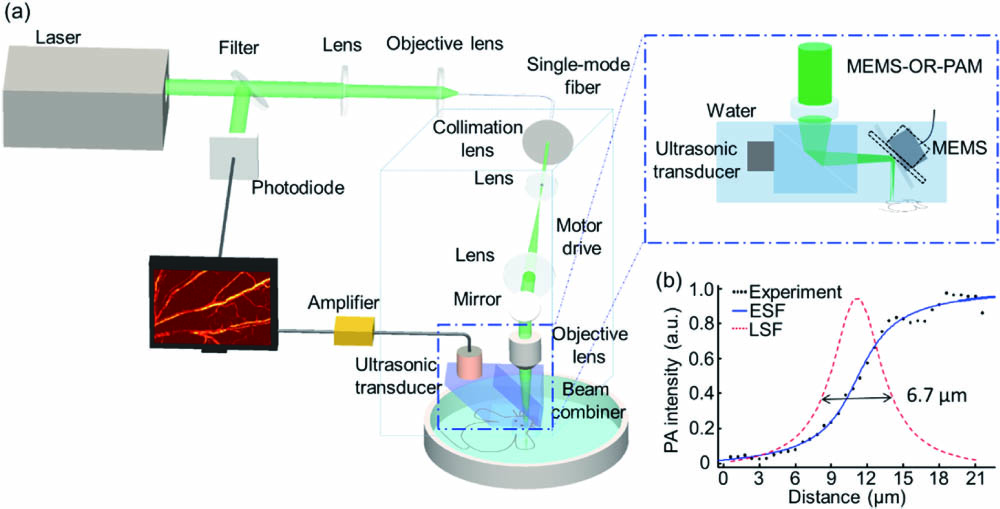
Fig. 1. OR-PAM system. (a) OR-PAM and MEMS-OR-PAM system scheme. (b) The ESF and LSF of a sharp edge.
As can be seen in Fig.
The RAW 264.7 cell line was chosen as the experiment cells to observe immune activities in inflammation, because it was well-characterized with regard to macrophage-mediated immune, metabolic, and phagocytic functions[24]. First of all, the commercial ink (Hu-Kaiwen ink) was filtered with a 0.22 μm filter and then added to the petri dish and incubated with the cells for 24 h (10% ink, 90% medium). The growth of the cells was not inhibited. The petri dish was washed with phosphate buffered saline (PBS) three times in order to wash off the free ink on the cells’ surface and the dish. Finally, the labeled cells were obtained by digestion centrifugation and made into suspension for in vivo imaging.
Balb/c nude mice were purchased from the experimental animal center of Xiamen University (5–8 weeks, 18 g). In the course of the experiment, the operating standards of experimental animals were strictly followed. OR-PAM was used to image the normal mouse ear. Then, the inflammation model was established by applying 20 μL xylene to the mouse ears evenly. Four groups were examined, including a normal ear control group, normal ear group injected with labeled macrophages, modeled ear group, and modeled ear group injected with labeled macrophages.
We chose the region of interest in the signal and background areas and then calculated the average intensity of them as the signal-to-background ratio. We quantified vessel parameters by calculating vessel density, average diameter, ratio, and average PA intensity[20]. From the original PAM images, two-dimensional (2D) blood vessels with linear structure were enhanced by Hessian filtering[25] and binarized by using threshold method to separate the signal from the background. The skeleton was obtained by eroding the vessel edge, and then the skeleton length was calculated to quantify the density. We used eight adjacent pixels’ field of each point on the vascular skeleton as the target to calculate the length. The length divided by two was considered as the actual vessel length. For the diameter, we obtained the skeleton normal of each point in the skeleton curve and calculated the length between the first two intersections of the normal with the binary vessel as the diameter of each vessel. The vessel diameter is defined by the average extracted diameter. The sum of the blood vessel intensity over the number of pixels in the image was defined as the average signal intensity. The vessel ratio was calculated by the signal pixels divided by the total pixels in the binarization image.
As shown in Fig.
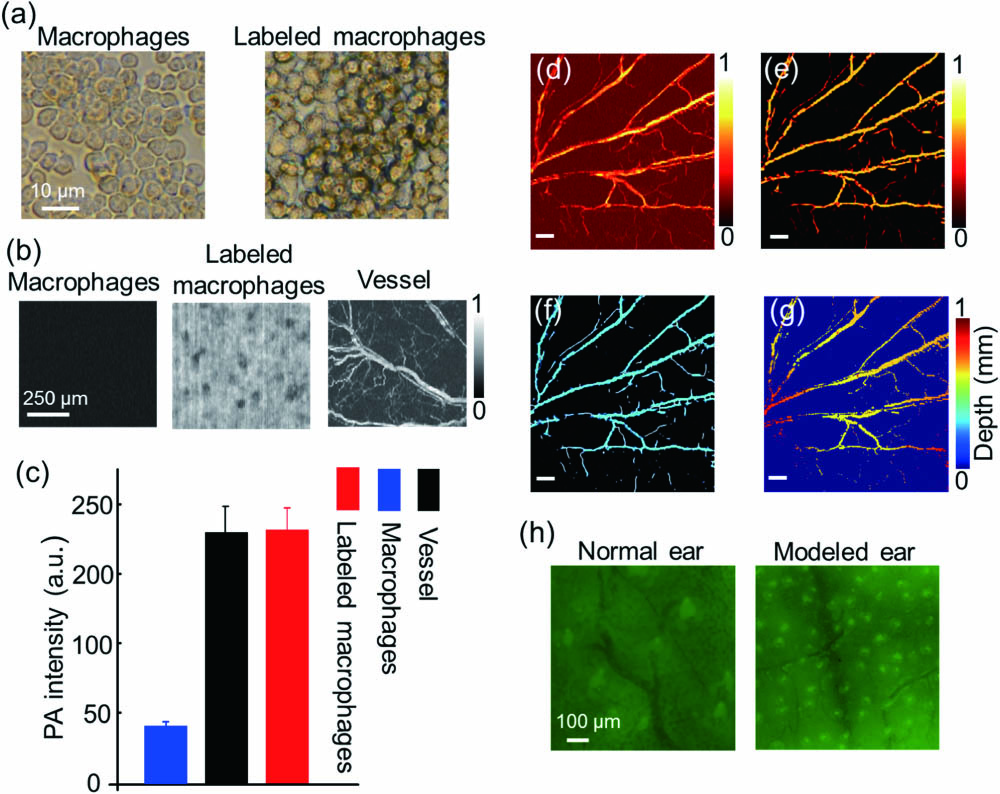
Fig. 2. Processing method of macrophage and vessel. (a) The optical microscopy images of labeled macrophages and macrophages. (b) The PA images of labeled macrophages, macrophages, and vessel by OR-PAM. (c) The PA intensities of labeled macrophages, macrophages background, and vessel. (d) The PA image of a normal mouse ear. (e) The PA image after the Hessian filter. (f) The centerline of the blood vessels. (g) The depth distribution of the PAM image. The scale bar is 500 μm. (h) The fluorescence images of normal and modeled mouse ears injected with macrophages.
We obtained PA images of the normal mouse ear group injected with labeled macrophages, modeled ear group, and modeled ear group injected with labeled macrophages by OR-PAM. Because the model is with acute inflammation, it can recover automatically in a short time. In order to observe macrophages activities after inflammation, we injected macrophages through the tail vein before modeling. We chose 10 min as the time interval to observe the macrophages movement throughout inflammation and an imaging range of . Each group consisted of at least three mice for statistical analysis. Edema may occur during the experiment, but it does not affect the PA imaging results since water absorbs very little light.
In the control group without modeling and macrophages [Fig.
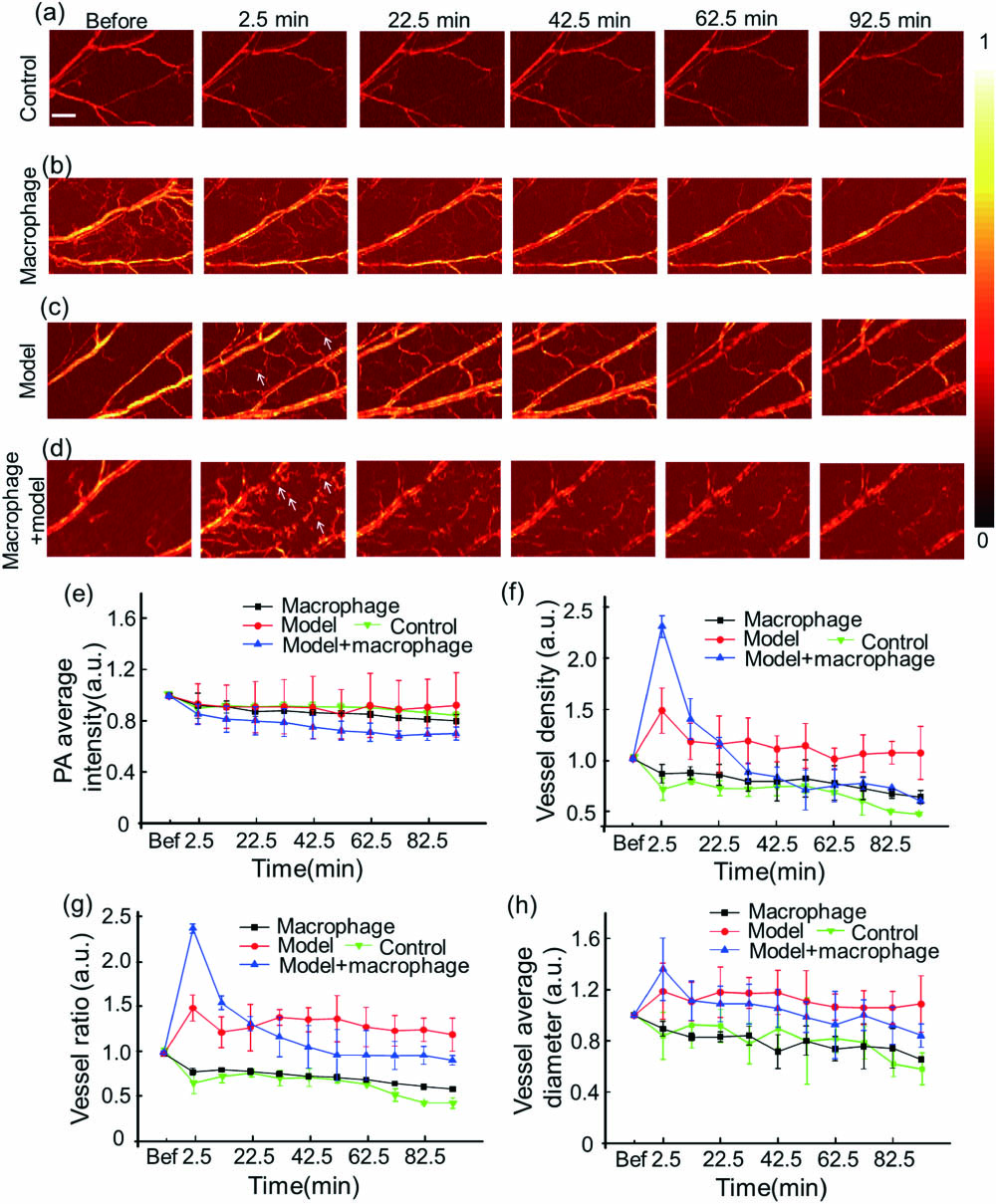
Fig. 3. Monitoring the vessel using OR-PAM. PA images of the (a) control group without modeling and macrophages, (b) normal mouse ear group injected with labeled macrophages, (c) modeled mouse ear group, and (d) modeled mouse ear group injected with labeled macrophages. (e) PA average intensity, (f) vessel density, (g) vessel ratio (the area of vessels signal/the area of the whole imaging), and (h) vessel average diameter of four groups. The white arrows point to the new vessels caused by inflammation, while the blue arrows represent labeled macrophages. The scale bar is 500 μm.
In the modeled mouse ear group [Fig.
In the modeled mouse ear group injected with macrophages [Fig.
Quantitative parameters of blood vessels also help to assess the macrophage activities. These data of each group were normalized to the average values before, because it can mitigate the issue of sample differences among different groups. The 2D structure reconstructed by a maximum amplitude projection algorithm was used for quantitative analysis[19]. There was no remarkable difference in the average PA intensity of blood vessels among the four groups [Fig.
For the average diameter of blood vessels [Fig.
In order to monitor the movement of macrophages more precisely after inflammation, the acquisition interval was set to 2 min in the modeled mouse ear injected with macrophages group by MEMS-OR-PAM with a small range, because MEMS had an imaging speed of 16 s per area [Fig.
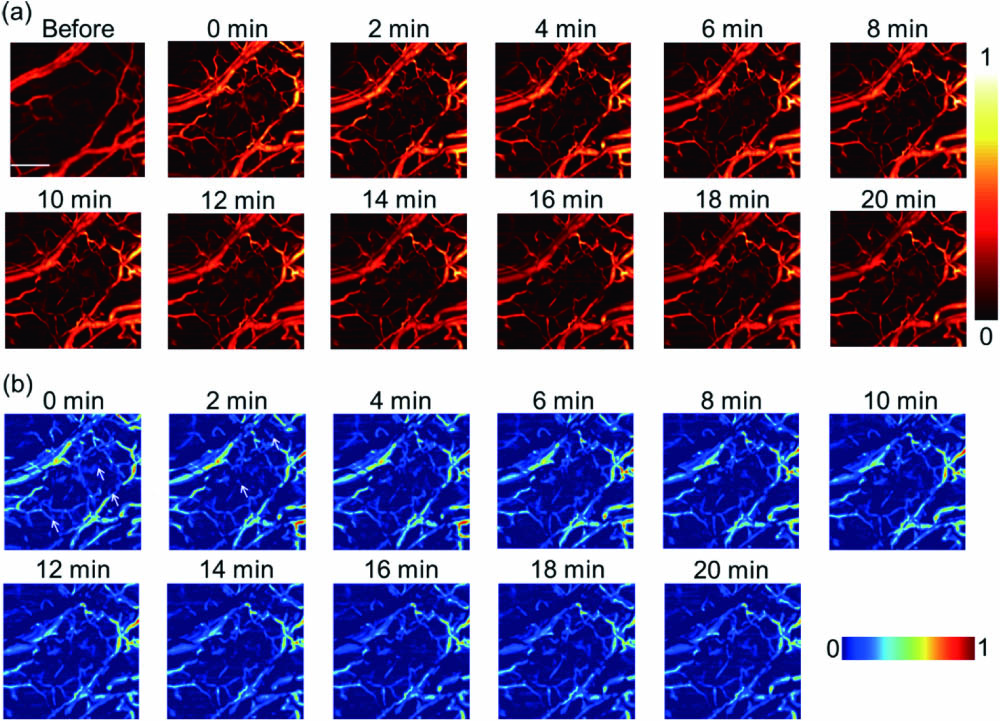
Fig. 4. Monitoring the vessel changes by MEMS-OR-PAM through 20 min. (a) PA images of the modeled mouse ear group injected with macrophages in 20 min. (b) The images were obtained by subtracting the ear before modeling from the modeled ear injected with macrophages. The arrows represent the dispersion labeled macrophages. The scale bar is 500 μm.
An OR-PAM system was built in our lab, which had a 6.7 μm lateral resolution. By the technique, the macrophage activities in inflammation were clearly visualized. In the modeled mouse ear injected with the macrophages group, some diffuse signals were detected around the vessels, and the main vessels’ diameter increased. After 62.5 min, the dotted signals disappeared, and the changes of vessels returned to the original state; however, no similar phenomenon was observed in other groups. From the quantitative data, the changes of vascular parameters returned to the initial state at last, while those in the modeled mouse ear group did not recover. In the acute inflammation, the macrophages tended to accumulate in the vessels first and then exuded into tissues to participate in phagocytosis of inflammatory cells. With the organism recovered from this situation, the cells gradually withdrew because they had less prone aggregation. Finally, they disappeared in this area until the immune behaviors ended. In summary, we can precisely trace the immune cells activities of inflammation with OR-PAM. This study will provide a new imaging method to study in vivo immune cells activities. In this work, we focused on blood vessels and cells activity in a large inflammation area in vivo. Our OR-PAM system, which achieved high-resolution imaging at a rapid imaging speed, can well meet both demands. This study will provide a new imaging method to study in vivo immune cells activities. In brief, the high-resolution system permits accurate information of macrophages activities in a rapid way.
[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
[12]
[13]
[14]
[15]
[16]
[17]
[18]
[19]
[20]
[21]
[22]
[23]
[24]
[25]
Fei Duan, Haosong Ma, Jinde Zhang, Shi Li, Honghui Li, Zhiyou Wu, Fengqiu Hong, Lüming Zeng, Liming Nie. Optical-resolution photoacoustic microscopy continually monitors macrophages activities of acute inflammation in vivo[J]. Chinese Optics Letters, 2020, 18(12): 121701.





