激光辐照下生物组织内光分布理论模型
1 引言
激光与组织相互作用机理的研究是一项十分复杂且应用广泛的基础性研究,随着激光技术的发展,很多应用需要对不同生物组织内的激光剂量进行定量分析,如激光医学[1-5]、激光外科[2]、激光热疗[6-7]及激光成像[4]等方面的应用。人们对于**领域的研究主要集中于对非致命激光**的人体效应评估等方面,例如对人眼的暂时性致盲以及对人体皮肤的热痛刺激等[8-9],而所有这些研究及应用的都是对激光辐照下组织内部光分布的准确描述。多年来该领域学者在无数理论和实验基础上试图通过多种数学及物理模型来准确描述组织内光的传播特性,国外方面,Jasiński[10]、Hamdy[11]、Gökçe[12]、Korczak[13]等诸多学者都对研究该问题的特定方法进行了深入的研究,但是能够较为系统地总结目前主流方法的综述类文章却很少,对这些方法的横向对比分析的文章更是匮乏;国内方面也面临着同样的问题,早期张震西等翻译的国外著作对该领域具有奠基性作用,后来以李小霞[14]为代表的天津大学的学者较早地对该问题展开了研究,此后王亚芬[15]、张纪庄[16]、董晓曦[17]和王晗[18]等都对该问题进行了一定的回顾。但通过大量分析国内外文献可以发现,目前几乎没有能够系统性地总结组织内光分布的主要理论、研究模型及定量分析方法的文献。因此很有必要对目前最新的理论及应用进行综述。研究该类问题的关键在于通过建立激光与组织间关系的数学模型来求解光在强散射且不均匀的活体组织介质中的传播和分布变化规律,因此本文主要对不同的理论模型进行总结及对比分析,并对该理论的前沿应用进行概述,以方便读者在全面了解该领域问题的同时快速追踪前沿。
2 生物组织中的光子传输
研究光分布模型的前提是对光子在生物组织中传输特性的准确描述。激光的物理剂量以及组织的光学特性参数共同决定了组织内部的光分布,利用光分布以及组织的热物学参数表示组织的热源,进而通过辐射传热方程求解组织内的温度场分布。温度场随时间的变化会改变组织的光学特性进而改变光分布,因此组织光热过程既是一个多物理场的耦合过程也是随时间变化的动态过程,总体研究思路如
表 1. 全文公式中的物理量含义
Table 1. Meaning of physical quantities in equations in this paper
|
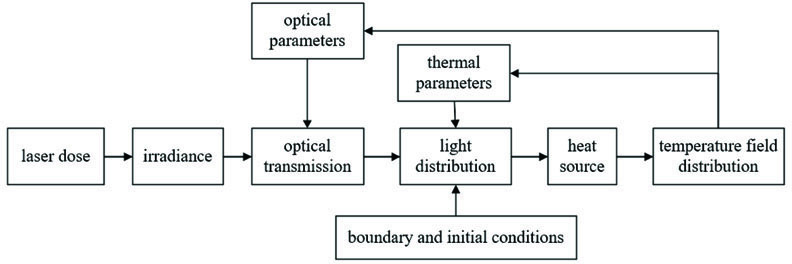
图 1. 生物组织光热效应的动态分析模型
Fig. 1. Dynamic analysis model of photothermal effect of biological tissues
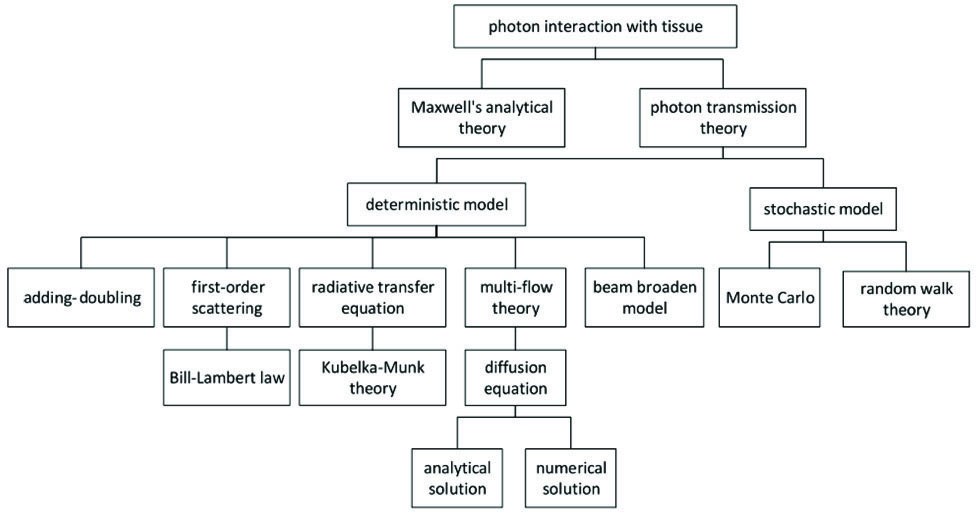
根据近似程度的不同,多年以来各国学者提出了多种方法来对光在生物组织内的传播行为进行理论建模,传输理论和解析理论是目前两种最主要的方法。解析理论从光的波动性出发,通过Maxwell方程组推导出相关变量的微积分方程,其在数学上的推导是严格的,但是需要得到组织的电介质特性且推导过程比较复杂,因此局限性较大。而传输理论描述了光子通过浑浊介质时的现象,因此没有上述这些限制,但这一理论具有一定的试探性特点,虽然不具备分析理论的严格性,但在大多数情况下光子传输理论的分析结果与实验结果的相关度很高,是目前国内外研究中应用最多的理论模型。
传输理论是从光的粒子性出发,依据光在混浊介质中的传播特性建立微分控制方程。Ishimaru[19]提出的辐射传输方程(RTE)便是对光子传输理论的详细描述,该方程描述了光子束辐照的空间变化[20]:
该方程的解为[21]
式中:Jc=J-Jd;δ
3 基于MC的光分布模型
3.1 分析方法
MC法是所有组织光学建模方法中最为准确的一种,具有不受组织结构复杂程度的限制、可以兼容高散射和低散射介质、可模拟混浊介质中非均匀性和折射率变化等特性的优势。在计算量可以承受的前提下,MC法能够以任何所需要的精度求解输运方程,为复杂结构的混浊介质中的光传输问题提供了一种灵活而严谨的计算方法。MC具有统计方法的灵活性,不仅可以用来求解正问题,也可以用来求解反问题。正问题通常是对给定光学性质的组织进行光分布模拟,而反问题则是通过将MC方法模拟的光分布与实验测量值进行拟合来估计组织光学性质。MC法最先由Metropolis和Ulam[
3.2 加速方法
目前主要通过5种加速方法来改善MC在速度方面的缺陷,分别为比例MC(SMC)、混合MC(HMC)、微扰MC(PMC)、减方差技术以及并行计算MC等方法。
3.2.1 比例MC
SMC法基于散射特性决定光子路径而吸收特性只影响存活光子权重这一事实,通过记录单个或多个基线MC光子的生存历史,应用比例关系来估计具有不同光学特性组织模型的漫反射或透射。该方法由Graaff等[29]率先提出,并快速计算了具有不同光学特性的平板状组织的总反射率和透射率,Kienle和Patterson[55]对于Graaff理论进行了扩展,以模拟具有任意光学特性的半无限均匀组织模型的空间和时间分辨漫反射,但该方法的离散表示和插值都可能引入误差,这些误差在计算时往往会被放大。Pifferi等[56]为此又提出了一种类似的方法,与Kienle和Patterson所提方法不同的是,Pifferi所提方法通过利用MC模拟结果在不同散射系数范围内的插值并通过对μa进行缩放来计算反射率和透射率。该方法提高了不同μs下结果的准确性,但代价是显著增加了基线MC模拟的数量。以上几位学者所提出的方法虽然速度快,但都无法避免离散和插值引入的误差。为了提高精度,Alerstam等[57]通过对单个光子进行比例运算实现了对Kienle和Patterson所提方法的改进;后来Palmer等[58]通过结合比例和卷积运算将Graaff等的定标方法从铅笔束照明扩展到光纤照明;Wang等[59]提出用两个卷积公式计算单光束在半无限介质中漫反射的方法。但这些方法都只涉及均匀的组织模型,Liu等[60]提出了一种将比例方法应用于多层组织模型的方法,并且该方法对两层和三层上皮组织模型进行了验证。近年来Jiang[61]、Sabzevari[62]和Rearden[63]等诸多学者都基于SMC方法解决了不同领域的实际问题。
3.2.2 微扰MC
PMC方法同样需要一次基线模拟以分析每个光子的出射权重、路径长度和碰撞次数的轨迹等信息,可给出基线模拟中的存活权重与微扰理论中的存活权重之间的关系[64-65],这使得该方法对每一次微扰的近似是有效的[66]。PMC法与SMC法相比在本质上是相似的,因此其精度取决于微扰模型中的新光学属性与基线模拟中的原始光学属性之间差异的大小,相反SMC本质上是精确的,因为它不考虑光学属性的差异,单纯的比例缩放不会产生近似现象。当扰动区域较小时,PMC的一个重要优势是简单和快速,因此广泛应用于光传输的反问题研究中。例如,Sassaroli等[64]提出了两个微扰关系,基于从均匀介质基线模拟中所获得的轨迹信息估计了引入散射/吸收不均匀介质的漫反射时间响应。Hayakawa等[65]证明了可将微扰关系与双参数Levenberg算法相结合,以快速解决双层组织模型中的光子逆迁移问题,并用这种方法从层状上皮组织模型中提取了光学特性[67]。Kumar和Vasu[68]提出了一种基于PMC法提取具有低μs的异质组织光学属性的新方法;Sassaroli[69]又提出了一种可用于在具有任意光学属性的组织模型中研究光子迁移的快速PMC方法,且该方法需要的硬盘空间最小,特别适用于解决像点变换等成像逆问题;Zhu和Liu [44]提出了一种结合SMC和PMC方法的混合方法,该方法与单独的SMC或PMC法相比,不仅具有速度上的优势也具有范围上的优势。Song等[70]提出了一种新的快速PMC法;Perfetti和Rearden[71]用连续能量的PMC法开发了用于灵敏度分析的广义理论;Leino等[72-73]将PMC法用于定量光声层析成像技术,这为该领域的研究带来了突破。
3.2.3 混合MC
HMC法是将其他较快速的方法耦合到标准MC模拟中,从而将MC在精度上的优势与其他方法在速度上的优势相结合,早期Duane等[74]对该方法进行了较为详细的概述。HMC法中最常见的是将标准MC与扩散理论相结合,例如Flock[75]、Wang[76]、Luo[77]等采用该方法解决了相关问题,他们首先对组织的光学性质和几何参数进行MC模拟,然后用MC模拟的结果对扩散理论的计算结果进行修正。HMC法不仅体现了MC法可在光源附近进行精确模拟的优势,也体现了扩散理论在远离光源位置的模拟速度优势。Wang[78]将该方法从半无限介质推广到有限厚度浊介质中;di Rocco等[79]采用HMC法加速了对深度不均匀的平板几何介质的MC模拟;Tinet等[80]将用于核工程领域的统计估计器应用于模拟时间分辨光散射问题的快速半解析MC模型之中;与Tinet等所提出的解法类似,Chatigny等[81]基于HMC法将乳腺组织模型分为多个各向同性和各向异性区域,有效地模拟了时间和空间透射。Alexandrakis等[82]提出了一种快速扩散的HMC方法,用于模拟双层人体皮肤模型中的空间分辨反射和相位延迟,并证明了HMC法比标准的MC模拟在速度上要快数百倍。近年来,Zhuang等[83]将基于能量区域划分的HMC法用于3D中子输运模拟之中;Prokhorenko等[84]用HMC方法完成了对结构和特性的预测;Lee和Mycek[85]通过带有射线跟踪的HMC法实现了混浊介质中的荧光测量。这些研究都充分证明了HMC法的独有优势和巨大潜力。
3.2.4 减方差技术
除了以上综述的混合方法外,最初应用于模拟中子输运的多重减方差技术[86]也已被用于组织中光输运的MC模拟。例如,加权光子模型和俄罗斯轮盘赌方案已经被用在多层组织中光子传输的MC建模中[87]。Liu和Ramanujam在MC建模中使用了最古老和使用最广泛的几何分裂减方差技术以加速MC数据库的创建,该方法将组织模型分成若干个域并通过在增加重要域中采样机会的同时减少在其他域中的采样机会来减小重要体积中的方差,根据模拟的漫反射估计了双层上皮组织模型的光学属性。Chen和Bai[88]提出了一种受控MC方法,引入了一个具有可调吸引因子的吸引点来使光子更有可能沿与探测器相交的方向传播,以此提高轨迹生成的效率,该方法可同时应用于透射几何[88]和反射几何[89]中。后来,Behin-Ain等[90]扩展了Chen的方法,有效地构建了由可见光或近红外光通过散射介质所产生的早期时间点扩展函数;Lima等[91-92]通过在标准MC中加入优化重要性抽样的方法,在时域OCT的快速MC仿真中获得了数百倍的加速。最近的研究中,Dubey等[93]提出了一种基于随机梯度Langevin动力学的减方差技术;Chatterji等[94]就关于随机梯度的MC减方差理论进行了较为详细的综述;Kilby等[95]基于MC的减方差技术解决了辐射传输层析成像问题。总而言之,4种加速方法都有各自独特的优势及局限,
表 2. 不同的加速MC方法的特点比较
Table 2. Comparison of characteristics of different accelerated MC methods
|
并行计算的MC法是单纯的并行加速计算机技术在MC运算中的应用,可以与上述4种方法相结合,此处不作详细叙述。除了SMC、PMC、HMC、减方差技术及并行计算MC等相对较成熟的方法之外,还有很多新方法被提出。Hardy等[97]建立了任意形状混浊介质中光子迁移的MC模型,将底层的数学形式转换为OpenCL语言代码,这使得在GPU上的模拟加速比大幅提高,从而进一步证明了并行加速这一观点;Talebi等[98]提出了基于MC模拟的优化快速数值方法,用多种算法来解决在任意对流边界条件和内部热源分布情况下的稳态和瞬态热传导问题;Marquet等[99]用两个概率分布函数模拟了混浊介质中的光分布以消除重复参数、提高运算效率。Graaff等[29]提出了一种压缩MC(CMC)算法来描述光传输,CMC模型的应用较为广泛。Lin等[100]用CMC法研究了具有任意μa和μs的混浊介质的空间分辨反射率,证明了该方法比传统的MC方法更加高效快速。Khalil等[101-103]提出反问题图解方法;van der Zee等[104-106]运用了一种在初步MC模拟之后生成查找表的方法来提高计算效率。
总而言之,MC方法除计算时间较长之外并无严重的缺陷,在很多方面具有其他方法都无法替代的优势,随着生物组织模型的复杂程度、精细程度以及耦合程度的不断提高,MC法势必展现出无限的潜力。此外,随着计算机辅助算法的不断成熟,MC法在计算时间上的瓶颈必然会被突破,因此越来越受到广大学者的青睐。
4 基于输运方程的光分布模型
4.1 光束加宽模型
光束加宽模型是基于光传输过程中的相干辐射度呈指数衰减所推导出来的,该模型将内部热源作用分为散射和吸收两部分。用数学方法描述组织的内部热源,可以较为合理地描述光在组织中的分布。该理论认为只有组织对光的吸收作用会导致光能量减小,从而产生热效应,散射作用只会造成光束在向前过程中的加宽。Grossweiner等[107-110]的研究表明,激光器中发出的激光可以被认为呈高斯分布,对从Boltzmann传输方程中得出的方程进行近似,可以得到
式中:μaφ
可以看出,利用已知激光参数及组织光学特性参数可描述空间中任一点的光分布,组织吸收作用所产生的热源项可表示为(4)式与μa的乘积。该模型很早就被应用于输运方程以研究生物介质[30,111],比如用基于该模型的光子密度波法可以检测肿瘤或者测量组织光学参数等[27]。该方法得到了国内外学者的广泛应用[112],李小霞等[113]较早地对该模型进行了深入研究并进行了大量的实验,Alexandrakis等[82,114]将生物组织看成一个线性系统,通过将入射光强与格林函数进行卷积,推导出了有限宽光束在圆柱坐标系下的传输过程并给出了光传输方程,从而克服了将入射光束假设为无限细所带来的局限性。近年来关于光束加宽模式的应用也趋于成熟,例如Li等[115]建立了皮肤的两层结构模型,计算了高斯分布的激光热源下模型的温度响应,从而研究了组织焊接应用于组织缝合线置换技术中时存在的问题;2017年Wang等[116]在经典漫射方程的基础上用该方法对多层组织的热源项描述进行了总结,得出了多层生物组织内部各层光分布的通用表达式。总而言之,该方法的优点在于在某些特定情况下可快速给出解析解[27],成为了描述近似高前向散射介质中光分布的最强大解决方案。
4.2 一阶散射理论
一阶散射理论[117]将辐射传输方程及其解简化为Beer-Lambert定律(BLL)的简单情况,所以入射激光束在光轴上z点的光强可由BLL表示为
一阶散射的基本假设是漫射强度远小于相干强度,多次散射的影响并未被充分考虑,因此该方法只局限于平面入射波,这样的解法仅在光学深度z≪1或反照率远小于0.5时可用。这种情况在光学深度相当小的光学诊断中是常见的;相反,高散射的波段(600~1300 nm)会造成光学深度z远小于1的假设不成立,因此利用一阶解可能不会得到精确的结果[14]。即便如此,一阶散射法在生物医学领域中仍然具有十分广泛的应用,例如Boas等[118]基于该方法并通过实验研究了混浊介质中光子与球形粒子的相互作用,从而解决了在多个散射中心的情况下进行成像的问题;Patterson等[119]基于混浊介质中的辐射输运方程,利用数学模型对生物组织中的光通量分布进行了研究;Li等[120]用低功率激光器采集厚生物组织的二维图像,通过该方法测量超声调制柱光透射后激光散斑图样的形成。近年来计算机技术的发展也推动了研究者对一阶散射理论的深入应用,例如,Jasi
4.3 光束扩散模型
光束扩散模型(BSF)[121]认为扩散程度是由μs和z决定的[122],它考虑了通过不同路径长度的高阶光子的影响,近似计算了浑浊介质中的光分布,而且它还可用于计算光强度的时间色散,该方法中格林函数是脉冲源在混浊介质中单向传播的解析近似。Beck和Teboulle[123]建立了该方法的加速梯度搜索法以求解μs和g。铅笔束的BSF在精度相当的情况下比基于统计学的MC法的计算时间要短得多。此外,该模型不但可以应用于均匀光源,也适用于高斯光束等其他光束,散射光子分布ksc
式中:τ=t-z/c是多径时间;h
归一化的时间色散分布函数G
式中:μ和σ分别是τ的一、二阶矩,且仅取决于散射角θ余弦的一、二阶矩。对ksc
总的来说,BSF模型将散射光子ksc
4.4 漫射近似理论
基于漫射近似理论进行研究的前提是输运方程中的漫射项不再具有各向异性[125],通常情况下可用μs'将各向异性散射转变为各向同性散射来进行计算[19],因此漫射近似的定义为
I=Aexp(-μtz)+Bexp(-μeffz), (11)
式中:A+B=I。当设置不同的μ、μs和g值时,漫射近似计算可以得到相似的辐照度,相似关系可表示为
漫射近似理论使得运算变得相当容易,只需要知道生物组织的μa以及μ's即可表示生物组织的光学特性,因此漫射近似理论在该领域研究中有着广泛的应用和不可替代的价值[54,126-128],尤其是在生物医学中应用广泛,例如可用于测量薄样品的光学特性[129]、红细胞的光散射[130]、人体组织的漫反射[131],可进行漫反射光学层析成像[132],可提高吸收介质中散射模型的准确性[133],用于分析组织光学活检的光散射的分形机制[134]以及具有非散射区的光学层析成像[135]等多个方面。Contini等[125]还对半无限扩散介质的含时扩散近似进行了分析,结果表明,折射率的影响对于μa和μs的表达式是不能忽略的;Karagiannes等[136]在宽光谱范围内测量了动植物组织的光学参数,测量结果与标准结果很好地吻合。此外,该方法也可以用来模拟均匀脂内体模的实验间隙辐射数据,例如Grabtchak等[137]利用此方法测定了650~900 nm范围内光的吸收和散射。当应用中需要激光脉冲持续一定的时间并达到一定的形状,或者需要激光脉冲在某个时间达到强度阈值时,可以使用RTE的漫射近似进行分析,例如,Morales-Cruzado等[138]发现在短脉冲入射的情况下使用RTE的漫射近似来计算变形脉冲的解析表达式,有利于从传输脉冲中恢复介质的光学特性。很多组织光热效应的模型都是采用漫射方程来求解激光辐照下的内部热源[139],例如,Yona[124]、Liu[140]、Jasi
总而言之,不同的模型有各自的优势和局限,具体方法的选择取决于组织的光学性质、光源的参数以及所需要的精度。
表 3. 定量分析方法的分类及特性对比
Table 3. Classification and characteristic comparison of quantitative analysis methods
|
5 结束语
主要对目前国内外研究激光辐照下组织内部光分布问题的数学模型进行了综述,通过对大量文献的总结提炼,发现目前该领域研究中所面临的几个主要问题。
1)系统研究动态性不足。在理论方面,目前几乎所有的理论都忽略了活体组织的自我调控以及对外界的应激反应等因素对组织光学性质造成的影响;在实验方面,目前绝大部分研究采用离体组织样本,而离体样本的血流灌注率及初始温度对结果造成的影响难以忽略,这些假设及限制都会给需要精确定量分析激光剂量的相关问题带来较大的误差。
2)波段研究规律性不足。目前的研究大多集中于常用波段的激光与组织的相互作用,人们对于很多其他波段的激光在组织中的光学和热物学性质不够了解,对于不同波段的激光在浑浊介质中传播的规律性研究更是欠缺,因此今后需要细化对特定组织中非常用波段的特异性模型并探索不同波段在相同浑浊介质中传播的规律。
3)光学模型功能涌现性不足。在计算机模型方面,很多研究存在边界条件设置不合理等问题,例如用无效的一维假设来忽略损失[143];在实体模型方面,目前大部分复合模型的不同部分还是相对简单的组合,较差的耦合性使其不具备良好的系统性,因此整体系统所带来的功能难以发挥。此外,一些具有复杂结构及异形结构的组织模型的精细程度不足,其真实解剖特点难以体现。
在今后的研究中,需要从以下几个方面进行改进:一是要加强对光热参数标准性的研究。虽然历年来无数的学者对组织光热参数进行了大量的研究及总结性工作[144-145],但是却没有形成一个完整的系统标准可供读者参考,而且保密等因素导致很多参数不够可靠,此外还存在国内外参数不一致[146-147]以及间接引用不准确[14,148]等问题,因此今后的研究中需要加强对于现有数据的甄别、统计及总结,进而建立一套具备准确性、权威性及系统性的光学参数标准体系。二是要加强多物理场耦合性研究,激光-组织的相互作用是热交互、光化学反应、光烧蚀、光激发、等离子体诱导消融及光中断等多种物理现象的耦合过程[149],因此必须要考虑它们的耦合作用所带来的影响。今后需要进一步研究各种物理现象对组织光分布的贡献,从宏观动态系统的角度探索可以更加准确解释生物组织等浑浊介质内光传输的优化模型。三是新兴技术应用性研究,例如3D生物打印技术[150-151]、一体化仿真技术[152]、机器学习、深度学习[153]及人工智能等,还可利用成像技术以及Mimics等软件工具,基于更加真实的组织结构建立更加精细化的模型,以提高光学模型的微观性、系统性,这都将会极大地提高研究者在建模仿真、实验研究以及数据分析方面的能力和速度,提高研究的准确性及效率。
[4] Yun S H, Kwok S J J. Light in diagnosis, therapy and surgery[J]. Nature Biomedical Engineering, 2017, 1: 0008.
[6] 马一鸣, 马立勇, 秦泽政, 等. 基于光声温度精准调控的光热治疗方法[J]. 中国激光, 2020, 47(10): 1007001.
[7] 丁乐明, 戴丽娟, 张磊, 等. 基于蒙特卡罗法的组织内插光纤出射激光的传输[J]. 中国激光, 2020, 47(2): 0207040.
[8] 濮立, 蒋贤沛, 刘小华, 等. 低能激光武器对人体皮肤组织的热损伤效应研究[J]. 激光杂志, 2016, 37(8): 13-17.
[9] Zhang FX, Han ZX, HaoW, et al. Research on thermal damage effect of a new laser weapon on human skin[C]∥2nd International Conference on Artificial Intelligence and Engineering Applications, September 23, 2017. Wuhan: Wuhan Zhicheng Times Culture Development Co. Ltd., 2017.
[10] Jasiński M. Modelling of thermal damage in laser irradiated tissue[J]. Journal of Applied Mathematics and Computational Mechanics, 2015, 14(4): 67-78.
[14] 李小霞. 激光照射下生物组织热效应的数值分析与实验研究[D]. 天津: 天津大学, 2004.
Li XX. Numerical analysis and experimental research on laser induced thermal effect in bio-tissues[D]. Tianjin: Tianjin University, 2004.
[15] 王亚芬. 1064 nm激光消融生物组织光、热场分布[D]. 上海: 上海交通大学, 2013.
Wang YF. Light and temperature distribution by laser ablation under 1064 nm[D]. Shanghai: Shanghai Jiao Tong University, 2013.
[16] 张纪庄. 皮肤病治疗中激光蚀除和选择性光热解的光热作用研究[D]. 北京: 清华大学, 2009.
Zhang JZ. Photo-thermal interactions of laser ablation and selective photothermolysis during laser treatments of skin diseases[D]. Beijing: Tsinghua University, 2009.
[17] 董晓曦. 双波长激光痛觉刺激的方法和技术研究[D]. 北京: 北京协和医学院, 2014.
Dong XX. Research on the method and technology of dual-wavelength laser pain stimulation[D]. Beijing: Peking Union Medical College, 2014.
[18] 王晗. 激光热痛刺激引起的皮肤组织温度分布的研究[D]. 北京: 北京协和医学院, 2017.
WangH. Study on the temperature distribution in skin tissue induced by laser based on thermal pain stimulation[D]. Beijing: Peking Union Medical College, 2017.
[19] Ishimaru A. Diffusion of light in turbid material[J]. Applied Optics, 1989, 28(12): 2210-2215.
[20] Bonner R F, Nossal R, Havlin S, et al. Model for photon migration in turbid biological media[J]. Journal of the Optical Society of America A, 1987, 4(3): 423-432.
[21] Luo Q, Gong H, Liu X. Simulation and inspection of laser transport in biological tissue[J]. Applied Optics, 1995, 24(2): 125-129.
[24] Sun X Q, Li X S, Ma L. A closed-form method for calculating the angular distribution of multiply scattered photons through isotropic turbid slabs[J]. Optics Express, 2011, 19(24): 23932-23937.
[25] Welch AJ, van Gemert M J C. Optical-thermal response of laser-irradiated tissue[M]. Dordrecht: Springer Netherlands, 2011.
[26] Prahl S A, Welch A J. Determining the optical properties of turbid media by using the adding-doubling method[J]. Applied Optics, 1993, 32(4): 559-568.
[27] 徐可欣, 高峰, 赵会娟. 生物医学光子学[M]. 2版. 北京: 科学出版社, 2011.
Xu KX, GaoF, Zhao HJ. Biomedical photonics[M]. 2nd ed. Beijing: Science Press, 2011.
[28] Metropolis N, Ulam S. The Monte Carlo method[J]. Journal of the American Statistical Association, 1949, 44(247): 335-341.
[29] Graaff R. Koelink M H, de Mul F F M, et al. Condensed Monte Carlo simulations for the description of light transport[J]. Applied Optics, 1993, 32(4): 426-434.
[30] Groenhuis R A J, Ferwerda H A, Ten Bosch J J. Scattering and absorption of turbid materials determined from reflection measurements. 1: theory[J]. Applied Optics, 1983, 22(16): 2456-2462.
[31] Meier R R, Lee L S, Anderson D E. Atmospheric scattering of middle UV radiation from an internal source[J]. Applied Optics, 1978, 17(20): 3216-3225.
[32] Qu J Y. MacAulay C E, Lam S, et al. Laser-induced fluorescence spectroscopy at endoscopy: tissue optics, Monte Carlo modeling, and in vivo measurements[J]. Optical Engineering, 1995, 34(11): 3334-3344.
[33] Tuchin VV. Handbook of optical biomedical diagnostics[M]. Bellingham: SPIE Press, 2002.
[34] Jacques SL, Wang LH. Monte Carlo modeling of light transport in tissuesoptical-thermal response of laser-irradiated tissue[M]. New York: Springer, 1995: 73- 100.
[35] WangL, Jacques SL. Monte Carlo modeling of light transport in multi-layered tissues in standard C[M]. Houston: University of Texas, 1992: 4- 11.
[38] Meglinsky I V, Matcher S J. Modelling the sampling volume for skin blood oxygenation measurements[J]. Medical and Biological Engineering and Computing, 2001, 39(1): 44-50.
[40] Lin Y, Northrop W F, Li X. Markov chain solution of photon multiple scattering through turbid slabs[J]. Optics Express, 2016, 24(23): 26942-26947.
[43] Cook P D, Bixler J N, Thomas R J, et al. Prediction of tissue optical properties using Monte Carlo modeling of photon transport in turbid media and integrating spheres (Conference Presentation)[J]. Proceedings of SPIE, 2020, 11238: 112380P.
[46] Terziev V. Modeling of temperature distribution in biotissues[J]. International E-Journal of Advances in Social Sciences, 2019, 5(14): 731-737.
[47] Chen B, Zhang Y, Li D. Numerical investigation of the thermal response to skin tissue during laser lipolysis[J]. Journal of Thermal Science, 2018, 27(5): 470-478.
[48] Burhan M T, Tozburun S. Monte-Carlo based simulations of photothermal response of nerve tissue for laser wavelengths of 1455 nm, 1490 nm, 1550 nm[J]. Proceedings of SPIE, 2020, 11238: 1123814.
[51] Ash C, Dubec M, Donne K, et al. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods[J]. Lasers in Medical Science, 2017, 32(8): 1909-1918.
[52] HamdyO, YoussefD, El-AzabJ, et al.Detection of breast diseases using numerical study of light propagation[C] ∥2018 9th Cairo International Biomedical Engineering Conference (CIBEC), December 20-22, 2018, Cairo, Egypt.New York: IEEE Press, 2018: 53- 56.
[53] Tuchin VV. Tissue optics[C] ∥Society of Photo-Optical Instrumentation Engineers,Bellingham: SPIE Press, 2015.
[54] Yaroslavsky I V, Yaroslavsky A N, Goldbach T, et al. Inverse hybrid technique for determining the optical properties of turbid media from integrating-sphere measurements[J]. Applied Optics, 1996, 35(34): 6797-6809.
[55] Kienle A, Patterson M S. Determination of the optical properties of turbid media from a single Monte Carlo simulation[J]. Physics in Medicine and Biology, 1996, 41(10): 2221-2227.
[56] Pifferi A, Taroni P, Valentini G, et al. Real-time method for fitting time-resolved reflectance and transmittance measurements with a Monte Carlo model[J]. Applied Optics, 1998, 37(13): 2774-2780.
[60] Liu Q, Ramanujam N. Scaling method for fast Monte Carlo simulation of diffuse reflectance spectra from multilayered turbid media[J]. Journal of Biomedical Optics, 2007, 17(1): 010501.
[62] Sabzevari I, Sharma S. Improved speed and scaling in orbital space variational Monte Carlo[J]. Journal of Chemical Theory and Computation, 2018, 14(12): 6276-6286.
[63] Rearden BT, Jessee M A. SCALE code system[EB/OL].(2016-04-01)[2020-07-14]. https:∥www.osti.gov/biblio/1424483.
[64] Sassaroli A, Blumetti C, Martelli F, et al. Monte Carlo procedure for investigating light propagation and imaging of highly scattering media[J]. Applied Optics, 1998, 37(31): 7392-7400.
[69] Sassaroli A. Fast perturbation Monte Carlo method for photon migration in heterogeneous turbid media[J]. Optics Letters, 2011, 36(11): 2095-2097.
[70] Song Y M, Li J W, Cai F H. Fast perturbation Monte Carlo simulation for heterogeneous medium and its utilization in functional near-infrared spectroscopy[J]. Journal of Physics: Conference Series, 2016, 680: 012019.
[71] Perfetti C M, Rearden B T. Development of a generalized perturbation theory method for sensitivity analysis using continuous-energy Monte Carlo methods[J]. Nuclear Science and Engineering, 2016, 182(3): 354-368.
[74] Duane S, Kennedy A D, Pendleton B J, et al. Hybrid Monte Carlo[J]. Physics Letters B, 1987, 195(2): 216-222.
[76] Wang L, Jacques S L. Hybrid model of Monte Carlo simulation and diffusion theory for light reflectance by turbid media[J]. Journal of the Optical Society of America A, 1993, 10(8): 1746-1752.
[77] Luo B, He S L. An improved Monte Carlo diffusion hybrid model for light reflectance by turbid media[J]. Optics Express, 2007, 15(10): 5905-5918.
[80] Tinet E, Avrillier S, Tualle J M. Fast semianalytical Monte Carlo simulation for time-resolved light propagation in turbid media[J]. Journal of the Optical Society of America A, 1996, 13(9): 1903-1915.
[81] Chatigny S, Morin M, Asselin D, et al. Hybrid Monte Carlo for photon transport through optically thick scattering media[J]. Applied Optics, 1999, 38(28): 6075-6086.
[82] Alexandrakis G, Farrell T J, Patterson M S. Monte Carlo diffusion hybrid model for photon migration in a two-layer turbid medium in the frequency domain[J]. Applied Optics, 2000, 39(13): 2235-2244.
[87] Wang L H, Jacques S L, Zheng L Q. MCML: Monte Carlo modeling of light transport in multi-layered tissues[J]. Computer Methods and Programs in Biomedicine, 1995, 47(2): 131-146.
[89] Chen N G. Controlled Monte Carlo method for light propagation in tissue of semi-infinite geometry[J]. Applied Optics, 2007, 46(10): 1597-1603.
[91] Lima I T, Kalra A, Sherif S S. Improved importance sampling for Monte Carlo simulation of time-domain optical coherence tomography[J]. Biomedical Optics Express, 2011, 2(5): 1069-1081.
[92] Lima I T, Kalra A. Hernández-Figueroa H E, et al. Fast calculation of multipath diffusive reflectance in optical coherence tomography[J]. Biomedical Optics Express, 2012, 3(4): 692-700.
[93] Dubey A, Reddi S J, Póczos B, et al. Variance reduction in stochastic gradient Langevin dynamics[J]. Advances in Neural Information Processing Systems, 2016, 29: 1154-1162.
[94] Chatterji NS, FlammarionN, Ma YA, et al. (2018-02-01)[2020-07-14]. http:∥www.publish.ac.cn/ArticleSubmit/Index/d9cd0022-ff1b-4ba3-8e8f-2f13a2e1ca2a/%E7%A8%BF%E4%BB%B6%E5%BE%85%E5%A4%84%E7%90%86.
[96] Hayashi T, Kashio Y, Okada E. Hybrid Monte Carlo-diffusion method for light propagation in tissue with a low-scattering region[J]. Applied Optics, 2003, 42(16): 2888-2896.
[97] Hardy L A, Chang C H, Myers E M, et al. Laser treatment of female stress urinary incontinence: optical, thermal, and tissue damage simulations[J]. Proceedings of SPIE, 2016, 9689: 96891R.
[104] van der Zee P. Methods for measuring the optical properties of tissue samples in the visible and near infrared wavelength range[J]. Proceedings of SPIE, 1993, 10311: 103110B.
[107] Grossweiner L I, Karagiannes J L, Johnson P W, et al. Gaussian beam spread in biological tissues[J]. Applied Optics, 1990, 29(3): 379-383.
[108] Grossweiner L I. Al-Karmi A M, Johnson P W, et al. Modeling of tissue heating with a pulsed Nd∶ YAG laser[J]. Lasers in Surgery and Medicine, 1990, 10(3): 295-302.
[109] Loze M K, Wright C D. Temperature distributions in semi-infinite and finite-thickness media as a result of absorption of laser light[J]. Applied Optics, 1997, 36(2): 494-507.
[113] 李小霞, 范世福, 赵友全. CO2激光照射活体皮肤的光热效应研究[J]. 光电子·激光, 2005, 16(10): 1257-1260.
Li X X, Fan S F, Zhao Y Q. Research on photo-thermal effect of in vivo skin irradiated by CO2 laser[J]. Journal of Optoelectronics· Laser, 2005, 16(10): 1257-1260.
[114] 王吉晖, 丁艳, 陈松林, 等. 有限宽光束在生物组织中传输的蒙特卡罗方法[J]. 光子学报, 2014, 43(S1): 167-171.
Wang J H, Ding Y, Chen S L, et al. Transport for photon beams of finite size in biological tissues based on Monte Carlo[J]. Acta Photonica Sinica, 2014, 43(S1): 167-171.
[117] IshimaruA. Isotropic scattering[M] ∥Wave Propagation and Scattering in Random Media. Amsterdam: Elsevier, 1978: 220- 233.
[118] Boas D A. O'Leary M A, Chance B, et al. Scattering of diffuse photon density waves by spherical inhomogeneities within turbid media: analytic solution and applications[J]. Proceedings of the National Academy of Sciences, 1994, 91(11): 4887-4891.
[119] Patterson M S, Chance B, Wilson B C. Time resolved reflectance and transmittance for the noninvasive measurement of tissue optical properties[J]. Applied Optics, 1989, 28(12): 2331-2336.
[121] McLean J W, Freeman J D, Walker R E. Beam spread function with time dispersion[J]. Applied Optics, 1998, 37(21): 4701-4711.
[123] Beck A, Teboulle M. A fast iterative shrinkage-thresholding algorithm for linear inverse problems[J]. SIAM Journal on Imaging Sciences, 2009, 2(1): 183-202.
[124] YonaG, MeitavN, KahnI, et al. and analytical modeling of light scattering in brain tissue for optogenetic applications[J]. eNeuro, 2016, 3(1): ENEURO. 0059-15. 2015.
[125] Contini D, Martelli F, Zaccanti G. Photon migration through a turbid slab described by a model based on diffusion approximation. I. Theory[J]. Applied Optics, 1997, 36(19): 4587-4599.
[127] Yaroslavsky A N, Schulze P C, Yaroslavsky I V, et al. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range[J]. Physics in Medicine and Biology, 2002, 47(12): 2059-2073.
[131] Sun P, Yang R Q, Xie F H, et al. A method for determining optical properties of human tissues by measuring diffuse reflectance with CCD[J]. Proceedings of SPIE, 2010, 7845: 784522.
[133] Cong A X, Shen H, Cong W, et al. Improving the accuracy of the diffusion model in highly absorbing media[J]. International Journal of Biomedical Imaging, 2007, 2007: 38168.
[136] Karagiannes J L, Zhang Z Y, Grossweiner B, et al. Applications of the 1-D diffusion approximation to the optics of tissues and tissue phantoms[J]. Applied Optics, 1989, 28(12): 2311-2317.
[139] Jasiński M. Numerical analysis of soft tissue damage process caused by laser action[J]. AIP Conference Proceedings, 2018, 1922(1): 060002.
[141] Majchrzak E, Jasiński M, Turchan Ł. Modeling of laser-soft tissue interactions using the dual-phase lag equation: sensitivity analysis with respect to selected tissue parameters[J]. Defect and Diffusion Forum, 2017, 379: 108-123.
[144] Lister T, Wright P, Chappell P. Optical properties of human skin[J]. Journal of Biomedical Optics, 2012, 17(9): 090901.
[145] Jacques S L. Optical properties of biological tissues: a review[J]. Physics in Medicine and Biology, 2013, 58(11): R37-R61.
[147] 陈荣, 黄宝华, 王月云, 等. 皮肤的光学模型[J]. 激光生物学报, 2005, 14(6): 520-526.
Chen R, Huang B H, Wang Y Y, et al. The optical model of human skin[J]. Acta Laser Biology Sinica, 2005, 14(6): 520-526.
[148] 王亚芬, 白景峰. 基于蒙特卡罗光传输算法的激光消融生物组织温度场分布计算[J]. 中国医疗器械杂志, 2013, 37(4): 252-254, 280.
[149] Guan K W, Jiang Y Q, Sun C S, et al. A two-layer model of laser interaction with skin: a photothermal effect analysis[J]. Optics & Laser Technology, 2011, 43(3): 425-429.
[152] Wang W M, Gibbon P, Sheng Z M, et al. Integrated simulation approach for laser-driven fast ignition[J]. Physical Review E, 2015, 91(1): 013101.
Article Outline
吕晨阳, 战仁军. 激光辐照下生物组织内光分布理论模型[J]. 激光与光电子学进展, 2021, 58(6): 0600003. Lü Chenyang, Zhan Renjun. Theoretical Models of Light Distribution in Biological Tissues Irradiated by Laser[J]. Laser & Optoelectronics Progress, 2021, 58(6): 0600003.