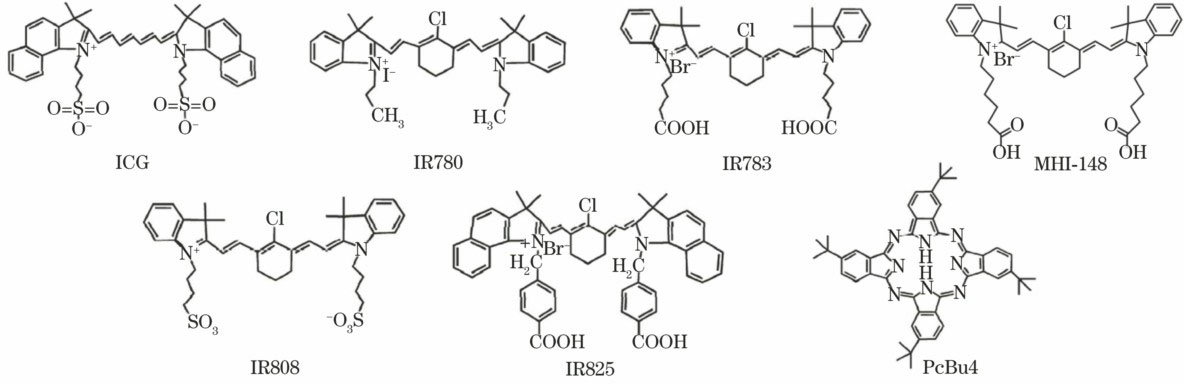
中国激光, 2018, 45 (2): 0207020, 网络出版: 2018-02-28
用于肿瘤光热治疗的有机纳米材料研究进展  下载: 1624次特邀综述
下载: 1624次特邀综述
Progress in Organic Nanomaterials for Laser-Induced Photothermal Therapy of Tumor
引用该论文
梁国海, 邢达. 用于肿瘤光热治疗的有机纳米材料研究进展[J]. 中国激光, 2018, 45(2): 0207020.
Liang Guohai, Xing Da. Progress in Organic Nanomaterials for Laser-Induced Photothermal Therapy of Tumor[J]. Chinese Journal of Lasers, 2018, 45(2): 0207020.
参考文献
[52] Liu T W. MacDonald T D, Shi J Y, et al. Intrinsically copper-64-labeled organic nanoparticles as radiotracers[J]. Angewandte Chemie International Edition, 2012, 51(52): 13128-13131.
梁国海, 邢达. 用于肿瘤光热治疗的有机纳米材料研究进展[J]. 中国激光, 2018, 45(2): 0207020. Liang Guohai, Xing Da. Progress in Organic Nanomaterials for Laser-Induced Photothermal Therapy of Tumor[J]. Chinese Journal of Lasers, 2018, 45(2): 0207020.