脑机接口技术的基础研究:神经元与二极管  下载: 733次封面文章
下载: 733次封面文章
The human brain as along with the entire nervous system comprises one of the most sophisticated biological systems, which has been the result of billions of years of evolution. Currently, our understanding of complex brain functions and structures is still at a rudimentary stage. Neural activities are closely associated with animal behaviors, including sensing, motion, emotion, learning, and memory, and are closely related to various neurological disorders and diseases. The modulation and detection of neural activities at the cellular, circuit, and behavior levels with high precision and spatiotemporal resolution have been the key objectives for advanced brain-machine interfaces, and they have substantial impacts on both fundamental neuroscience studies and medical therapeutics. Over the past few decades, progress has been made in the development of a kaleidoscope for materials, devices, and systems for neural modulation and sensing using electrical, optical, thermal, acoustic, and magnetic signals.
In the past few years, research has been primarily focused on the development of microscale implantable optoelectronic devices for advanced optical neural interfaces. In this review, we provide an overview of our recent efforts, focusing on the fundamental elements of brain-machine interfaces, that is, neurons and diodes. We demonstrate that semiconductor diodes that can realize the conversion of electrical and optical signals, which can function as an interface to interact with biological signals, realizing the detection and regulation of neural activities (Fig. 1).
Our discussion is divided into the following four parts:
(1) Light-emitting diodes (LEDs) can generate light signals and optogenetically modulate neural activity (Fig. 2). We developed a wireless, dual-color optogenetic probe for the manipulation of bidirectional neuronal activity. The flexible probe comprises vertically assembled, thin-film microscale LEDs, which have colocalized red and blue emissions and enable chronic in vivo operations with desirable biocompatibility. In synergy with the co-expression of two spectrally distinct opsins (ChrimsonR and stGtACR2), red or blue irradiations deterministically activate or silence the neurons. In a mouse model, the probe interferes with dopaminergic neurons in the ventral tegmental area, thereby increasing or decreasing the dopamine levels. Such bidirectional regulation further generates rewarding and aversive behaviors and interrogates social interactions among multiple mice.
(2) Silicon-based photodiodes can convert light into electrical signals by activating and inhibiting neural activity (Fig. 3). Thin-film monocrystalline silicon p-n diodes can establish polarity-dependent positive or negative photovoltages at the semiconductor/solution interface. Under laser illumination, the silicon-diode optoelectronic interfaces enable the deterministic depolarization or hyperpolarization of cultured neurons as well as upregulated or downregulated intracellular calcium dynamics. Moreover, optoelectronic interfaces can be mounted on nerve tissue to either activate or silence neural activities in peripheral and central nervous tissues, as demonstrated in mice with exposed sciatic nerves and somatosensory cortices. Finally, these thin-film silicon devices naturally dissolve in biological environments and exhibit desirable biocompatibility.
(3) An implantable photodiode can detect fluorescence signals in the brain (Fig. 4). An injectable fluorescence photometer powered by a wirelessly operated circuit integrates a miniaturized LED and a photodiode on a flexible needle, which is suitable for injection into the deep brain of mice. The system enables wireless stimulation and recording of fluorescence associated with genetically encoded calcium indicators, with unique capabilities for visualizing neuronal activity. The ultrathin geometry and compliant mechanics of these probes enable minimally invasive implantation and stable chronic operation. In vivo studies involving freely moving animals have demonstrated that this technology enables high-fidelity recording of calcium fluorescence in the deep brain, with measurement characteristics that match or exceed those associated with fiber photometry systems.
(4) A semiconductor diode can sense the electrical signals of neurons (Fig. 5). An LED simultaneously absorbs and emits photons, thus enabling wireless power harvesting and signal transmission. Additionally, owing to its strong photon-recycling effects, its photoluminescence (PL) emission exhibits a superlinear dependence on external conductance. These unique mechanisms can be exploited to optically monitor instantaneous biophysical signals, such as galvanic skin response, demonstrating that such an optoelectronic sensing technique outperforms a traditional tethered electrically-based sensing circuit, particularly in terms of the footprint, accuracy, and sensitivity. Furthermore, we envision that such a photon-recycling mechanism can be leveraged for the optical detection of the electrophysiological signals of neurons on a large scale.
In this review, we introduce the interplay between the semiconductor diode (one of the fundamental elements of electronics) and the neuron (the fundamental element of neurology) and highlight our recent work in the past few years. We summarize our results of the design and fabrication of advanced semiconductor diodes for biological integration. These thin-film, microscale light-emitting diodes (LEDs) and photodetectors can integrate with biological cells, tissues, and organs to modulate and sense neural signals. Representative studies include the use of optoelectronic devices for optogenetic stimulation, wireless electrical stimulation, fluorescence detection, and biological sensing. These advanced bio-integrated optoelectronic devices offer broad potential for fundamental neuroscientific studies and clinical applications.
1 引言
以大脑为代表的神经系统是生物体最复杂、最精密的器官和系统,是人类历经千万年持续自然进化和筛选而获得的高效率、低功耗的“处理器+存储器”,已成为人工智能模仿的最佳模型,但人类对于大脑的认知还处于早期的初步探索阶段。神经信号的活动与感知、运动、情绪、学习记忆等行为密切相关,也与神经系统疾病紧密联系[1-4]。开发新型脑机接口的材料、器件与系统,运用电、光、热、磁、化学等多模态信号手段,对单个神经元或者特定神经网络的活动进行实时、精确的调控或检测,对于推进基础生物学研究、开发神经疾病治疗手段、实现高性能类脑计算系统等具有重要意义,已成为国内外学者关注的前沿热点[5-9]。近年来,多模态神经调控与检测技术的研究随着各国“脑计划”项目的大力投入[10-11]得到了迅猛发展。在机理研究层面,电、光、热、磁、化学等各种信号被探索并被应用于对离体和在体神经活动的调控与检测[12-15];在材料器件层面,各种生物友好的生物材料与器件以其柔性、延展性甚至可降解性的优化设计,通过穿戴、植入等方式与生物系统进行集成[5,16-20];在电路系统层面,新型的无线能量与信号传输策略不断涌现[21-25];在生物医疗层面,除了已被广泛应用的人工耳蜗、脑起搏器等技术之外,基于多模态信号的新型神经调控与检测技术,在癫痫治疗、神经义肢、视觉假体、神经疼痛治疗等临床应用中逐步开展验证[26-29]。
作为信息产业的基石,以硅基和化合物基半导体材料(砷化镓、氮化镓等)为基础设计的半导体光电器件,目前已被广泛应用于微电子芯片、光电探测与成像、光伏电池等系统中[30-31],这类器件具有尺寸微小、功能丰富和性能优异等优势。另外,凭借良好的生物相容性,薄膜半导体光电器件近年来也被用于与各种柔性、可延展甚至可降解衬底集成,进而实现应用于生物医疗领域的穿戴式和植入式传感器[32-33]。因此,开发基于光电半导体结构的新型脑机接口技术,可以探究神经元[生物脑组织的基本结构单元,如
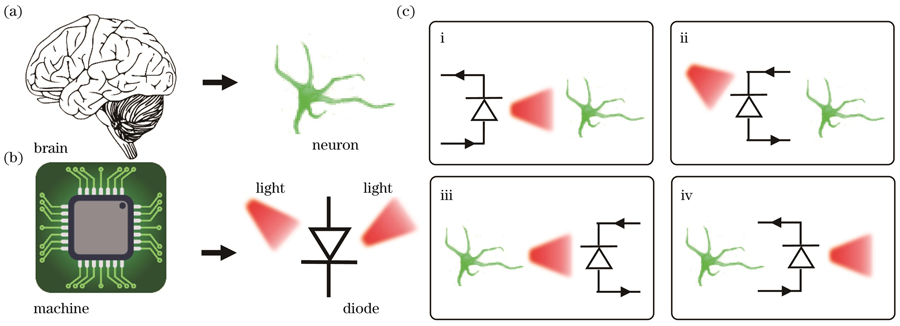
图 1. 脑机接口的基础科学问题:神经元与二极管的相互作用。(a)神经系统(“脑”)的基本单元——神经元;(b)信息技术(“机”)的基本单元——二极管;(c)利用光电二极管对神经元信号进行调控与检测:i.发光二极管(LED)在电驱动下产生光信号,对神经元进行光遗传学刺激;ii.光电二极管(PD)在光照下产生光致电场,对神经元进行电刺激;iii.表达有光敏蛋白质的神经元发出的特异性荧光信号被光电二极管采集,检测神经活动;iv.可调控发光二极管发光性能的神经元的电活动被成像系统采集后可检测神经活动
Fig. 1. Fundamental problem in the area of brain-machine interface: interaction between neurons and diodes. (a) Fundamental unit in the neural system (“brain”) is a single neuron; (b) fundamental unit in the information system (“machine”) is a semiconductor diode; (c) semiconductor diodes are utilized to modulate and detect neural activities: i. An electrically driven light-emitting diode (LED) can emit light to stimulate neural activities via optogenetics; ii. Under illumination, a photodiode (PD) can generate photoelectric signals to stimulate neural activities; iii. A photodiode can capture fluorescence signals generated from neurons expressing photosensitive protein, thus detecting neural activities; iv. The optical emission from an LED can be altered by the electrophysiological activities of neurons, which can be used for neural signal detecting
本文主要回顾了本课题组近几年的代表性工作:通过探索二极管与神经元之间的基本关系,开发新型神经调控与传感技术。根据光电二极管的伏安特性,对二极管在不同工作条件下的光电转换过程进行控制,可以实现对生物神经元的光遗传刺激、非遗传光电刺激、荧光信号采集、电生理信号放大等,如
2 基于双色LED探针的双向光遗传学调控技术
光遗传学是一种结合光学技术和遗传学基因技术的新型交叉技术,该技术通过在靶细胞或靶器官上表达光敏离子通道蛋白来获得用相应波长的光激活光敏离子通道的功能,从而借助光学手段实现细胞、组织、器官以及动物神经组织的精确调控。2006年,Deisseroth等[38]首次在神经细胞上应用光遗传学这一方法,随后这种方法凭借其优异的特异性、准确性以及较高的时空分辨率,推动了面向脑功能和神经疾病的生物学研究的快速发展[39-42]。不同于借助传统植入式光纤的光学传递手段[43],结合微型薄膜式发光器件(micro-LED)与无线控制电路的植入式光源进行光遗传学调控[23,44-46],不仅摆脱了有线方式的束缚,而且实现了多空间位点和多波长发光的功能,如
![用无线控制的双色微型LED探针对神经元活动进行双向光遗传学调控[34]。(a)探针植入小鼠目标脑区进行双色神经激活-抑制调控示意图;(b)细胞尺度下探针的光调控功能示意图:红光LED的开启打开了阳离子通道ChrimsonR,使细胞去极化;蓝光LED的开启打开了阴离子通道stGtACR2,使细胞超极化;(c)蓝光和红光薄膜微型LED垂直堆叠集成在柔性聚酰亚胺(PI)衬底上形成的双色LED探针的结构示意图,其中的滤光片用于实现光谱上的选择性反射或透过;(d)LED探针的显微照片,显示双色LED可独立控制发出蓝光和红光;(e)实验和仿真结果展示红光和蓝光在模拟脑组织中的传播示意图;(f)微型LED探针与无线电路结合,可独立发出蓝光或红光;(g)红光和蓝光交替发光进行激活和抑制时细胞活动的电生理记录;(h)红光和蓝光LED刺激下多巴胺信号强度的变化情况;(i)共表达stGtACR2和ChrimsonR蛋白的小鼠在红光或者蓝光刺激下对位置偏好或厌恶行为的运动热力图分析;(j)三只植入无线探针的小鼠自由活动的照片](/richHtml/zgjg/2023/50/9/0907301/img_02.jpg)
图 2. 用无线控制的双色微型LED探针对神经元活动进行双向光遗传学调控[34]。(a)探针植入小鼠目标脑区进行双色神经激活-抑制调控示意图;(b)细胞尺度下探针的光调控功能示意图:红光LED的开启打开了阳离子通道ChrimsonR,使细胞去极化;蓝光LED的开启打开了阴离子通道stGtACR2,使细胞超极化;(c)蓝光和红光薄膜微型LED垂直堆叠集成在柔性聚酰亚胺(PI)衬底上形成的双色LED探针的结构示意图,其中的滤光片用于实现光谱上的选择性反射或透过;(d)LED探针的显微照片,显示双色LED可独立控制发出蓝光和红光;(e)实验和仿真结果展示红光和蓝光在模拟脑组织中的传播示意图;(f)微型LED探针与无线电路结合,可独立发出蓝光或红光;(g)红光和蓝光交替发光进行激活和抑制时细胞活动的电生理记录;(h)红光和蓝光LED刺激下多巴胺信号强度的变化情况;(i)共表达stGtACR2和ChrimsonR蛋白的小鼠在红光或者蓝光刺激下对位置偏好或厌恶行为的运动热力图分析;(j)三只植入无线探针的小鼠自由活动的照片
Fig. 2. Colocalized, bidirectional optogenetic modulation of neural activities with a wireless dual-color micro-LED probe[34]. (a) Schematic view of probe inserted into target brain area to perform dual-color neural activation and inhibition; (b) schematic of cellular scale depiction of probe’s photo-modulation function: a red LED regulates cation channel ChrimsonR for depolarization, while a blue LED regulates anion channel stGtACR2 for hyperpolarization; (c) exploded view of dual-color LED probe made from vertically stacked blue and red thin-film micro-LEDs assembled on a flexible polyimide (PI) substrate, with a filter for spectrally selective reflection and transmission; (d) optical images of a micro-LED probe, showing dual-color LED can be independently controlled to realize blue and red emissions; (e) experimental photographs and simulated results illustrating blue and red light propagation in a brain phantom; (f) images of a micro-LED probe integrated with a wireless circuit for independent control of red and blue emissions; (g) cell activity traces presenting bidirectional spike activation and inhibition with alternating red and blue illuminations; (h) representative variations of dopamine signals in response to stimulation by red and blue LEDs; (i) representative heat maps showing real-time preference and aversion behavior following red or blue stimulation for mice co-expressing stGtACR2 and ChrimsonR; (j) photograph showing three free-moving mice implanted with wireless probes
针对表达ChrimsonR和stGtACR2这两种光敏蛋白质不同组合的同一神经元细胞,对其提供高精准的光激发工具是不可或缺的[34]。堆叠式无线双色微型薄膜式LED光源可以在同一大脑区域内产生红色和蓝色双光照射。与平行结构相比,堆叠结构中红光和蓝光的重叠辐射区域更大,为双向光遗传学的精确调控提供了足够的辐射范围。
利用双色LED微探针(交替发射红光和蓝光)对表达ChrimsonR和stGtACR2的神经元进行光遗传学调控,并借助膜片技术记录神经元细胞动作电位的变化过程,如
3 基于硅基二极管薄膜的无线光电神经调控技术
随着基因编码技术的发展,以光遗传学为代表的精准神经调控手段引起了国内外的广泛关注,这些手段展示了光学的高时空分辨率和最小的侵入性,为精确的特异性神经调节提供了一种强大的工具。然而,使用细胞靶向基因修饰会不可避免地阻碍光遗传学的直接临床应用。近年来,研究人员纷纷着眼于对光学介导的物理方式的探索,比如,水[51-52]、金属[53-54]、有机材料[55-62]、无机材料[63-67]、石墨单原子层材料[68-69]引起的光热、光电、光声和光电化学效应等,这些光诱导物理刺激也被用于非遗传学的无线化神经调节。基于光热的激活和抑制可能归因于热相关离子通道的瞬态大温升引起的激活效应以及相对缓慢的小温升引起的抑制效应[70-71]。在复杂的生物环境中,通常需要细微的、与强度和时间相关的热学管理,以防止细胞功能发生紊乱甚至不可逆的损伤[72-73]。另外,基于光电化学和光电容效应,可将光能转化成电能,直接与神经元、其他种类细胞(例如胶质细胞)发生作用。这些光电刺激方法在体外和体内都展现出了能够诱导神经兴奋的功能。然而,同样至关重要的非遗传学诱导神经抑制的功能[74]尚未得到充分开发。因此,尽管电诱导细胞去极化和超极化已经被很好地理解[75-76],但仍迫切需要发展以光电为基础的无线远程技术,以便实时、精确地调控神经的兴奋和抑制取向。作为常见的半导体硅基薄膜器件,pn二极管可以产生光诱导的正/负电场,以一种非遗传学无线光电策略来实现光诱发细胞的去极化和超极化,并调节细胞内的钙活动。此外,根据硅二极管的极性,这些光电接口还能在体内选择性地激发或抑制外周和中枢神经系统。这些硅薄膜可以在动物体内自然溶解,展现出了理想的生物相容性。
![用于神经活动光电激活或抑制的薄膜硅二极管[35]。(a)自支撑的硅薄膜(厚度约为2 μm)转移到柔性衬底上的照片;(b)光照下p+n和n+p型硅膜二极管产生不同极性的电信号;(c)电信号可以调节神经元膜电位的去极化(左)和超极化(右);(d)p+n和n+p型硅膜产生的光电信号激活和抑制神经元活动;(e)用473 nm激光照射贴在小鼠脑皮层上的硅膜,多通道记录探针采集脑皮层电信号;(f)p+n和n+p硅膜选择性地激活(上)和抑制(下)小鼠脑皮层电信号;(g)硅膜调控小鼠外周神经示意图,用光照射贴在坐骨神经上的硅膜;(h)植入下肢肌肉中的肌电(EMG)电极记录了不同强度脉冲光照下的复合肌肉动作电位(CMAPs)](/richHtml/zgjg/2023/50/9/0907301/img_03.jpg)
图 3. 用于神经活动光电激活或抑制的薄膜硅二极管[35]。(a)自支撑的硅薄膜(厚度约为2 μm)转移到柔性衬底上的照片;(b)光照下p+n和n+p型硅膜二极管产生不同极性的电信号;(c)电信号可以调节神经元膜电位的去极化(左)和超极化(右);(d)p+n和n+p型硅膜产生的光电信号激活和抑制神经元活动;(e)用473 nm激光照射贴在小鼠脑皮层上的硅膜,多通道记录探针采集脑皮层电信号;(f)p+n和n+p硅膜选择性地激活(上)和抑制(下)小鼠脑皮层电信号;(g)硅膜调控小鼠外周神经示意图,用光照射贴在坐骨神经上的硅膜;(h)植入下肢肌肉中的肌电(EMG)电极记录了不同强度脉冲光照下的复合肌肉动作电位(CMAPs)
Fig. 3. Thin-film silicon diodes for optoelectronic excitation and inhibition of neural activities[35]. (a) Image of a freestanding Si film (thickness of ~2 µm) transferred onto a flexible substrate; (b) optical illumination generates polarity-dependent electrical signals by p+n Si and n+p Si film diodes; (c) electrical signals can depolarize (left) and hyperpolarize (right) neuron membrane potentials; (d) photoelectric signals from p+n Si and n+p Si films activate and inhibit neural activities; (e) a multichannel recording probe is guided into mouse brain to sample extracellular activities by illuminating Si films attached on the mouse cortex with a 473 nm laser; (f) p+n Si and n+p Si films selectively elicit (top) and suppress (bottom) electrical signals on mouse cortex; (g) cartoon diagram illustrating the modulation of mice’s peripheral nervous, with light illuminating Si films attached on sciatic nerve; (h) compound muscle action potentials (CMAPs) are recorded by EMG recording electrode inserted into hindlimb-related muscles under pulsed light with various intensities
这个例子用薄膜硅二极管实现了对培养细胞、外周和中枢神经系统中非遗传学的神经活动的激发和抑制。这些硅薄膜可以应用于深层组织的无线、无电池刺激,可以采用近红外光源进行光学激活。此外,与其他基于Ⅲ‑V族和有机半导体的光电材料相比,薄膜硅基器件的另一个特性是它们在生物系统中具有良好的生物相容性甚至是可降解性,避免了二次手术带来的风险。相比于传统的电刺激方案,这种无线光电调控器件可以运用红外光进行远程无线操作,消除了引线和电路带来的限制,同时又避免了光遗传技术依赖的基因修饰问题,这些优点都显示了这种技术在医疗应用上的广阔前景。
4 基于微型光电器件的植入式荧光检测探针
随着生物光学标记技术的进步,特别是随着基因编码的荧光指示剂的发展[35,76-79],荧光计也被用于与生物体相结合,在活体动物脑组织中通过检测钙离子浓度、电势变化等引起的荧光蛋白发光强度的变化来监测动物的神经活动[80-81]。与传统的生物神经电生理信号测试用荧光指示剂相比,基因编码的荧光指示剂能够选择性地标定特定的神经细胞或者组织,在特定激发光下发出荧光信号,这种精准的、特异性的荧光标记能够将特定的神经元荧光信号与生物活动联系起来,对于深入理解生物体内的神经活动具有重要意义。传统的荧光计通常包含多个分立的光学元件,包括激发光源、聚焦透镜、单色仪(或者滤光片)、光电探测器等。通过对器件进行优化,这些荧光计可以达到很高的探测灵敏度(高达1×10-12)[82],但其体积较大[83],通常只适用于生物体外环境,难以应用于植入生物体内的活体荧光信号的检测。基于类似结构的光学系统可结合共聚焦、双光子等技术,应用于动物脑皮层的荧光检测与成像[84],但由于生物组织对光学信号的强烈散射和吸收作用,光信号难以进入深层组织(深度大于1 mm),应用这种非侵入式的光学系统很难从外界检测到深层脑组织的荧光信号。在神经科学中,活体动物的复杂行为研究(运动、社交等)也对便携式神经荧光检测系统提出了较高要求。因此,通过合理的微纳光电子器件和光学结构设计,制备生物相容的、小型化、高度集成化的植入式荧光计,成为实现生物体内信号稳定、高精度光学检测的重要手段[85],而这种对活动中的哺乳动物的神经活动进行记录的能力可以极大地扩展对大脑功能的理解。
在前期植入式光源微探针研究的基础上,更多薄膜、微型半导体器件和光学结构进一步集成至柔性探针上[86],用于获得组织内部光源的传递、荧光信号的提取和光电信号的转换等功能,如
![用于深部脑区钙荧光信号无线监测的植入式光电探针[36]。(a)在柔性衬底上集成InGaN微型LED以及GaAs光电探测器的无线植入式荧光检测探针结构示意图,其中的薄膜滤光片涂层用于波长选择性透过;(b)钙离子荧光检测探针的概念图;(c)左图是光电探针的光学显微照片,右图是探针尖端扫描电子显微照片的伪彩图;(d)无线探针系统的照片;(e)植入荧光探针的小鼠照片;(f)AAV-DJ-CaMKII-GCaMP6f(绿色)病毒在BLA脑区中注射示意图,以及钙荧光指示剂表达后的脑区切片荧光照片;(g)无线设备记录的小鼠在电刺激前后的钙荧光信号热力图(上),其在时间轴上与平均值(用曲线表示)±标准差(曲线附近的阴影)(下)对齐](/richHtml/zgjg/2023/50/9/0907301/img_04.jpg)
图 4. 用于深部脑区钙荧光信号无线监测的植入式光电探针[36]。(a)在柔性衬底上集成InGaN微型LED以及GaAs光电探测器的无线植入式荧光检测探针结构示意图,其中的薄膜滤光片涂层用于波长选择性透过;(b)钙离子荧光检测探针的概念图;(c)左图是光电探针的光学显微照片,右图是探针尖端扫描电子显微照片的伪彩图;(d)无线探针系统的照片;(e)植入荧光探针的小鼠照片;(f)AAV-DJ-CaMKII-GCaMP6f(绿色)病毒在BLA脑区中注射示意图,以及钙荧光指示剂表达后的脑区切片荧光照片;(g)无线设备记录的小鼠在电刺激前后的钙荧光信号热力图(上),其在时间轴上与平均值(用曲线表示)±标准差(曲线附近的阴影)(下)对齐
Fig. 4. Wireless optoelectronic probe for monitoring calcium fluorescence signal in the deep brain[36]. (a) Schematic exploded-view illustration of a wireless, injectable, ultrathin photometry probe with a InGaN micro-LED and a GaAs photodevice at flexible substrate, where thin-film filters serve as wavelength-selective coatings; (b) conceptual illustration of microprobe system for Ca2+ fluorescence sensing; (c) left image is optical micrograph of injectable photometry probe and right image is magnified colorized SEM image of probe tip; (d) photo of wirelessly operated probe system; (e) photograph of a mouse implanted with fluorescent probe; (f) injection schematic of virus AAV-DJ-CaMKII-GCaMP6f (green) into BLA and fluorescence photo of brain-area section expressed with calcium fluorescence indicator; (g) heatmap (upper) for Ca2+ fluorescence signals recorded by wireless device before and after shock, aligned with trace plotted as mean (curves) ± standard deviation (shading around curves) (lower)
5 基于光电二极管的无线光学生物传感
传统的可穿戴设备主要由金属电极、放大电路和电池等组件构成,并通过有线(电缆和光纤等)或者无线(蓝牙、无线网络或者近场通信等)等方式实现数据的交换和展示[87-89]。然而,复杂的电路设计、能源的供给方式和附加的信息显示,限制了传统可穿戴设计在生理信息传感应用上的微型化、便捷化和低功耗的拓展升级。不同状态的半导体光电器件二极管可具备光学能量采集(光伏效应)、电学信号放大(二极管的特性)、光学信号传输(通过光致发光传输)等不同的属性和功能[90-94]。借助高性能半导体光电器件的光子回收效应,可以在器件内部实现“光子”与“电子”之间的相互转换,同时使器件获得上述三种状态的一体化功能[95-97]。进一步,将生物信号作为变量因素引入高性能半导体光电器件的“光电-电光”转换过程中,可以实现单一器件结构对生物信号无线化传感的新设计。
![用于实现光学传感生物活动的半导体二极管[37]。(a)附着在人体皮肤上的薄膜红光微型LED示意图,用于基于电导相关的光子回收(PR)效应实现生物电信号的无线光学传感;(b)传感器的工作原理,显示不同外接电路状态(短路、开路、外接电阻R1>R2)下微型LED在绿光激发下的光致发光(PL)强度变化;(c)皮肤电反应(GSR)的光电传感示意图;(d)微型LED PL发射的显微图像,显示受试者在基础呼吸和深呼吸条件下微型LED的不同的PL强度;(e)基于半导体二极管光子回收效应的生物电信号光学读出示意图;(f)一个微型红光LED的典型电流-电压特性实验结果;(g)在不同驱动电压下得到的绝对(红色)和相对(蓝色)PL强度响应(相对于1 mV电压的变化)](/richHtml/zgjg/2023/50/9/0907301/img_05.jpg)
图 5. 用于实现光学传感生物活动的半导体二极管[37]。(a)附着在人体皮肤上的薄膜红光微型LED示意图,用于基于电导相关的光子回收(PR)效应实现生物电信号的无线光学传感;(b)传感器的工作原理,显示不同外接电路状态(短路、开路、外接电阻R1>R2)下微型LED在绿光激发下的光致发光(PL)强度变化;(c)皮肤电反应(GSR)的光电传感示意图;(d)微型LED PL发射的显微图像,显示受试者在基础呼吸和深呼吸条件下微型LED的不同的PL强度;(e)基于半导体二极管光子回收效应的生物电信号光学读出示意图;(f)一个微型红光LED的典型电流-电压特性实验结果;(g)在不同驱动电压下得到的绝对(红色)和相对(蓝色)PL强度响应(相对于1 mV电压的变化)
Fig. 5. A semiconductor diode is utilized to optically sense biological activities[37]. (a) Schematic of a thin-film and red-emitting micro-LED attached onto human skin for wireless optical sensing of biophysical signals based on its conductance dependent photon-recycling (PR) effect; (b) operational principle of the sensor, showing that the micro-LED’s photoluminescence (PL) intensity is dependent on its load resistances in different working conditions: short-circuit, open-circuit, and connected to different resistances (R1>R2); (c) schematic illustration of optoelectronic sensing of galvanic skin response (GSR); (d) microscopic images of micro-LED’s PL emission, showing different PL intensities of micro-LED under basal and deep breath conditions of a subject; (e) scheme showing optical readout of bioelectrical signals based on photon recycling of a semiconductor diode; (f) typical current-voltage characteristic measured for a red-emitting micro-LED; (g) absolute (red) and relative (blue) PL intensities in response to a voltage change of 1 mV, as a function of applied voltage
不同于通过外部阻值的变化间接影响光电二极管内部的光子回收过程,外部电压或者电流的变化通过直接影响半导体光电器件的光子回收效果来获得对神经元信号的光学传感功能。生物神经元的电信号处于μV至mV量级,可以借助半导体器件的光子回收效应对微小的电信号进行放大,从而获得对神经元信息的高灵敏度光学传感。
因此,在半导体光电器件的光电转换过程中引入神经组织的生理信息(电生理信号、电阻信号、电化学信号、离子浓度信号等),可以影响器件结构中载流子辐射复合的转换比例,最终使两者的相互作用过程呈现在发光强度的变化上。借助半导体器件的微纳加工技术,对离散的、笨重的、刚性的器件结构进行进一步集成化和功能化升级,可以获得性能优异、创伤小、生物亲和性高的诊疗工具。
6 结束语
本文总结了半导体光电信息科学与生物医疗交叉应用的前沿发展,介绍了不同形式和不同工作状态的半导体光电器件在生物医疗特别是在神经传感和调控方面的典型应用,针对器件的光电原理、结构设计、加工工艺和集成封装等进行探索。一方面,介绍了微型、薄膜式半导体光电材料与器件的研制,即:通过新型的光电器件工艺技术,在柔性、可延展、生物相容甚至可降解的衬底上制备光电器件,再将这些光电器件与生物体进行集成,使光子、电子信号与生物组织直接进行相互作用。将微型半导体光电器件制成植入式探针的形式作用于小鼠等动物脑内,可以精准定位特定脑区和神经核团,有效地将生物神经系统与光、电等物理信号进行耦合,实现对生物信号(光、电、热、力学、化学等信号)的检测和控制,已成为动物神经调控的重要工具。另一方面,从基本的光电子原理出发,探索光与材料、生物组织的相互作用,设计新型的光学结构,发展新型的光电子器件,优化先进器件的集成技术,与神经系统交叉融合,可以实现对生物信号(光、电、热、力学、化学等信号)的无线化传感。
未来,借助半导体光电器件所具有的能源供给、光学传递、信号放大、光电探测等一系列优势,深入探索光子、电子信号与神经元及群体的相互作用过程,可为神经调控和检测提供精准的工具和手段。开发新型光电子器件与异质衬底的集成技术,可为生物传感、医学诊断、疾病治疗等应用提供有效的工具支持,特别是为神经科学的研究和新一代脑机接口技术的发展提供新思路。
[1] Grassi G. Sympathetic neural activity in hypertension and related diseases[J]. American Journal of Hypertension, 2010, 23(10): 1052-1060.
[2] Hou Y N, Wu X M, Hallett M, et al. Frequency-dependent neural activity in Parkinson’s disease[J]. Human Brain Mapping, 2014, 35(12): 5815-5833.
[3] Yuan P, Zhang M Y, Tong L, et al. PLD3 affects axonal spheroids and network defects in Alzheimer’s disease[J]. Nature, 2022, 612(7939): 328-337.
[4] Oswal A, Cao C Y, Yeh C H, et al. Neural signatures of hyperdirect pathway activity in Parkinson’s disease[J]. Nature Communications, 2021, 12: 5185.
[5] Won S M, Song E, Reeder J T, et al. Emerging modalities and implantable technologies for neuromodulation[J]. Cell, 2020, 181(1): 115-135.
[6] Patel S R, Lieber C M. Precision electronic medicine in the brain[J]. Nature Biotechnology, 2019, 37(9): 1007-1012.
[7] Chen R, Canales A, Anikeeva P. Neural recording and modulation technologies[J]. Nature Reviews Materials, 2017, 2: 16093.
[8] 孔令杰, 靳程, 金国藩. 基于光遗传学的在体高空间分辨率神经调控技术[J]. 中国激光, 2021, 48(15): 1507003.
[9] 刘洋, 刘东远, 张耀, 等. 面向脑机接口应用的便携式fNIRS拓扑成像系统:全并行检测与初步范式实验[J]. 中国激光, 2021, 48(11): 1107001.
[10] Abbott A. How the world’s biggest brain maps could transform neuroscience[J]. Nature, 2021, 598(7879): 22-25.
[11] 蒲慕明, 徐波, 谭铁牛. 脑科学与类脑研究概述[J]. 中国科学院院刊, 2016, 31(7): 725-736, 714.
Pu M M, Xu B, Tan T N. Brain science and brain-inspired intelligence technology: an overview[J]. Bulletin of Chinese Academy of Sciences, 2016, 31(7): 725-736, 714.
[12] Frank J A, Antonini M J, Anikeeva P. Next-generation interfaces for studying neural function[J]. Nature Biotechnology, 2019, 37(9): 1013-1023.
[13] Hong G S, Lieber C M. Novel electrode technologies for neural recordings[J]. Nature Reviews Neuroscience, 2019, 20(6): 330-345.
[14] Kim M G, Kamimura H A S, Lee S A, et al. Image-guided focused ultrasound modulates electrically evoked motor neuronal activity in the mouse peripheral nervous system in vivo[J]. Journal of Neural Engineering, 2020, 17(2): 026026.
[15] Hong G S, Fu T M, Qiao M, et al. A method for single-neuron chronic recording from the retina in awake mice[J]. Science, 2018, 360(6396): 1447-1451.
[16] Chiong J A, Tran H, Lin Y J, et al. Integrating emerging polymer chemistries for the advancement of recyclable, biodegradable, and biocompatible electronics[J]. Advanced Science, 2021, 8(14): e2101233.
[17] Woods G A, Rommelfanger N J, Hong G S. Bioinspired materials for in vivo bioelectronic neural interfaces[J]. Matter, 2020, 3(4): 1087-1113.
[18] Lago N, Cester A. Flexible and organic neural interfaces: a review[J]. Applied Sciences, 2017, 7(12): 1292.
[19] Lu C, Park S, Richner T J, et al. Flexible and stretchable nanowire-coated fibers for optoelectronic probing of spinal cord circuits[J]. Science Advances, 2017, 3(3): e1600955.
[20] Choi S, Han S I, Jung D, et al. Highly conductive, stretchable and biocompatible Ag-Au core-sheath nanowire composite for wearable and implantable bioelectronics[J]. Nature Nanotechnology, 2018, 13(11): 1048-1056.
[21] Gutruf P, Krishnamurthi V, Vázquez-Guardado A, et al. Fully implantable optoelectronic systems for battery-free, multimodal operation in neuroscience research[J]. Nature Electronics, 2018, 1(12): 652-660.
[23] Wentz C T, Bernstein J G, Monahan P, et al. A wirelessly powered and controlled device for optical neural control of freely-behaving animals[J]. Journal of Neural Engineering, 2011, 8(4): 046021.
[24] Piech D K, Johnson B C, Shen K, et al. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication[J]. Nature Biomedical Engineering, 2020, 4(2): 207-222.
[25] Zhou A, Santacruz S R, Johnson B C, et al. A wireless and artefact-free 128-channel neuromodulation device for closed-loop stimulation and recording in non-human primates[J]. Nature Biomedical Engineering, 2019, 3(1): 15-26.
[26] Lee G H, Moon H, Kim H, et al. Multifunctional materials for implantable and wearable photonic healthcare devices[J]. Nature Reviews Materials, 2020, 5(2): 149-165.
[27] 史钊, 李丽珠, 赵钰, 等. 植入式生物医疗光电子器件与系统[J]. 中国激光, 2018, 45(2): 0207001.
[28] Liu X, Ren C, Lu Y C, et al. Multimodal neural recordings with neuro-FITM uncover diverse patterns of cortical–hippocampal interactions[J]. Nature Neuroscience, 2021, 24(6): 886-896.
[29] Baldo T A, de Lima L F, Mendes L F, et al. Wearable and biodegradable sensors for clinical and environmental applications[J]. ACS Applied Electronic Materials, 2021, 3(1): 68-100.
[30] Luo L B, Zhang X X, Li C, et al. Fabrication of PdSe2/GaAs heterojunction for sensitive near-infrared photovoltaic detector and image sensor application[J]. Chinese Journal of Chemical Physics, 2020, 33(6): 733-742.
[31] Blokhin S A, Sakharov A V, Nadtochy A M, et al. AlGaAs/GaAs photovoltaic cells with an array of InGaAs QDs[J]. Semiconductors, 2009, 43(4): 514-518.
[32] Kim D H, Lu N S, Ma R, et al. Epidermal electronics[J]. Science, 2011, 333(6044): 838-843.
[33] Hwang S W, Tao H, Kim D H, et al. A physically transient form of silicon electronics[J]. Science, 2012, 337(6102): 1640-1644.
[34] Li L Z, Lu L H, Ren Y Q, et al. Colocalized, bidirectional optogenetic modulations in freely behaving mice with a wireless dual-color optoelectronic probe[J]. Nature Communications, 2022, 13: 839.
[36] Lu L Y, Gutruf P, Xia L, et al. Wireless optoelectronic photometers for monitoring neuronal dynamics in the deep brain[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(7): E1374-E1383.
[37] Ding H, Lü G Q, Shi Z, et al. Optoelectronic sensing of biophysical and biochemical signals based on photon recycling of a micro-LED[J]. Nano Research, 2021, 14(9): 3208-3213.
[38] Deisseroth K, Feng G P, Majewska A K, et al. Next-generation optical technologies for illuminating genetically targeted brain circuits[J]. The Journal of Neuroscience, 2006, 26(41): 10380-10386.
[39] Wu F, Stark E, Ku P C, et al. Monolithically integrated μLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals[J]. Neuron, 2015, 88(6): 1136-1148.
[40] Zhang F, Wang L P, Brauner M, et al. Multimodal fast optical interrogation of neural circuitry[J]. Nature, 2007, 446(7136): 633-639.
[41] Boyden E S, Zhang F, Bamberg E, et al. Millisecond-timescale, genetically targeted optical control of neural activity[J]. Nature Neuroscience, 2005, 8(9): 1263-1268.
[42] Karl D. Optogenetics[J]. Nature Methods, 2011, 8(1): 26-29.
[43] Adamantidis A R, Zhang F, Aravanis A M, et al. Neural substrates of awakening probed with optogenetic control of hypocretin neurons[J]. Nature, 2007, 450(7168): 420-424.
[44] Kim T I, McCall J G, Jung Y H, et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics[J]. Science, 2013, 340(6129): 211-216.
[45] Park S I, Brenner D S, Shin G, et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics[J]. Nature Biotechnology, 2015, 33(12): 1280-1286.
[46] Montgomery K L, Yeh A J, Ho J S, et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice[J]. Nature Methods, 2015, 12(10): 969-974.
[47] Inoue K I, Takada M, Matsumoto M. Neuronal and behavioural modulations by pathway-selective optogenetic stimulation of the primate oculomotor system[J]. Nature Communications, 2015, 6: 8378.
[48] Ferenczi E A, Vierock J, Atsuta-Tsunoda K, et al. Optogenetic approaches addressing extracellular modulation of neural excitability[J]. Scientific Reports, 2016, 6: 23947.
[50] Frontera J L, Baba Aissa H, Sala R W, et al. Bidirectional control of fear memories by cerebellar neurons projecting to the ventrolateral periaqueductal grey[J]. Nature Communications, 2020, 11: 5207.
[51] Shi L L, Jiang Y, Fernandez F R, et al. Non-genetic photoacoustic stimulation of single neurons by a tapered fiber optoacoustic emitter[J]. Light: Science & Applications, 2021, 10: 143.
[52] Duke A R, Jenkins M W, Lu H, et al. Transient and selective suppression of neural activity with infrared light[J]. Scientific Reports, 2013, 3: 2600.
[53] Carvalho-de-Souza J L, Treger J S, Dang B B, et al. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles[J]. Neuron, 2015, 86(1): 207-217.
[54] Yoo S, Park J H, Nam Y. Single-cell photothermal neuromodulation for functional mapping of neural networks[J]. ACS Nano, 2019, 13(1): 544-551.
[55] Ghezzi D, Antognazza M R, Maccarone R, et al. A polymer optoelectronic interface restores light sensitivity in blind rat retinas[J]. Nature Photonics, 2013, 7(5): 400-406.
[56] Martino N, Feyen P, Porro M, et al. Photothermal cellular stimulation in functional bio-polymer interfaces[J]. Scientific Reports, 2015, 5: 8911.
[57] Jiang Y, Huang Y, Luo X, et al. Neural stimulation in vitro and in vivo by photoacoustic nanotransducers[J]. Matter, 2021, 4(2): 654-674.
[58] Rand D, Jakešová M, Lubin G, et al. Direct electrical neurostimulation with organic pigment photocapacitors[J]. Advanced Materials, 2018, 30(25): e1707292.
[59] Jakešová M, Silverå Ejneby M, Đerek V, et al. Optoelectronic control of single cells using organic photocapacitors[J]. Science Advances, 2019, 5(4): eaav5265.
[60] Silverå Ejneby M, Jakešová M, Ferrero J J, et al. Chronic electrical stimulation of peripheral nerves via deep-red light transduced by an implanted organic photocapacitor[J]. Nature Biomedical Engineering, 2022, 6(6): 741-753.
[61] Airaghi Leccardi M J I, Chenais N A L, Ferlauto L, et al. Photovoltaic organic interface for neuronal stimulation in the near-infrared[J]. Communications Materials, 2020, 1: 21.
[62] Han M, Srivastava S B, Yildiz E, et al. Organic photovoltaic pseudocapacitors for neurostimulation[J]. ACS Applied Materials & Interfaces, 2020, 12(38): 42997-43008.
[63] Parameswaran R, Carvalho-de-Souza J L, Jiang Y W, et al. Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires[J]. Nature Nanotechnology, 2018, 13(3): 260-266.
[64] Jiang Y W, Li X J, Liu B, et al. Rational design of silicon structures for optically controlled multiscale biointerfaces[J]. Nature Biomedical Engineering, 2018, 2(7): 508-521.
[65] Tang J, Qin N, Chong Y, et al. Nanowire arrays restore vision in blind mice[J]. Nature Communications, 2018, 9: 786.
[66] Jiang Y W, Carvalho-de-Souza J L, Wong R C S, et al. Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces[J]. Nature Materials, 2016, 15(9): 1023-1030.
[67] Han M, Bahmani Jalali H, Yildiz E, et al. Photovoltaic neurointerface based on aluminum antimonide nanocrystals[J]. Communications Materials, 2021, 2: 19.
[68] Rastogi S K, Garg R, Scopelliti M G, et al. Remote nongenetic optical modulation of neuronal activity using fuzzy graphene[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(24): 13339-13349.
[69] Savchenko A, Cherkas V, Liu C, et al. Graphene biointerfaces for optical stimulation of cells[J]. Science Advances, 2018, 4(5): eaat0351.
[70] Eom K, Byun K M, Jun S B, et al. Theoretical study on gold-nanorod-enhanced near-infrared neural stimulation[J]. Biophysical Journal, 2018, 115(8): 1481-1497.
[71] Carvalho-de-Souza J L, Pinto B I, Pepperberg D R, et al. Optocapacitive generation of action potentials by microsecond laser pulses of nanojoule energy[J]. Biophysical Journal, 2018, 114(2): 283-288.
[72] Wells J, Kao C, Konrad P, et al. Biophysical mechanisms of transient optical stimulation of peripheral nerve[J]. Biophysical Journal, 2007, 93(7): 2567-2580.
[73] Owen S F, Liu M H, Kreitzer A C. Thermal constraints on in vivo optogenetic manipulations[J]. Nature Neuroscience, 2019, 22(7): 1061-1065.
[74] Wiegert J S, Mahn M, Prigge M, et al. Silencing neurons: tools, applications, and experimental constraints[J]. Neuron, 2017, 95(3): 504-529.
[75] Schoen I, Fromherz P. The mechanism of extracellular stimulation of nerve cells on an electrolyte-oxide-semiconductor capacitor[J]. Biophysical Journal, 2007, 92(3): 1096-1111.
[76] Benfenati V, Toffanin S, Bonetti S, et al. A transparent organic transistor structure for bidirectional stimulation and recording of primary neurons[J]. Nature Materials, 2013, 12(7): 672-680.
[77] Zhao Y X, Araki S, Wu J H, et al. An expanded palette of genetically encoded Ca²⁺ indicators[J]. Science, 2011, 333(6051): 1888-1891.
[78] Akerboom J, Chen T W, Wardill T J, et al. Optimization of a GCaMP calcium indicator for neural activity imaging[J]. The Journal of Neuroscience, 2012, 32(40): 13819-13840.
[79] Chen T W, Wardill T J, Sun Y, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity[J]. Nature, 2013, 499(7458): 295-300.
[80] Warden M R, Cardin J A, Deisseroth K. Optical neural interfaces[J]. Annual Review of Biomedical Engineering, 2014, 16: 103-129.
[81] Lin M Z, Schnitzer M J. Genetically encoded indicators of neuronal activity[J]. Nature Neuroscience, 2016, 19(9): 1142-1153.
[82] Liu J W, Brown A K, Meng X L, et al. A catalytic beacon sensor for uranium with parts-per-trillion sensitivity and millionfold selectivity[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(7): 2056-2061.
[83] KhandpurR S. Fluorometer[M]∥Compendium of Biomedical Instrumentation. New York: John Wiley & Sons, Ltd., 2019: 823-827.
[84] Yun S H, Kwok S J J. Light in diagnosis, therapy and surgery[J]. Nature Biomedical Engineering, 2017, 1: 8.
[85] Warden M R, Cardin J A, Deisseroth K. Optical neural interfaces[J]. Annual Review of Biomedical Engineering, 2014, 16: 103-129.
[86] Liu C B, Zhao Y, Cai X, et al. A wireless, implantable optoelectrochemical probe for optogenetic stimulation and dopamine detection[J]. Microsystems & Nanoengineering, 2020, 6: 64.
[87] Gao W, Emaminejad S, Nyein H Y Y, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis[J]. Nature, 2016, 529(7587): 509-514.
[88] Chung H U, Kim B H, Lee J Y, et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care[J]. Science, 2019, 363(6430): eaau0780.
[89] Bariya M, Nyein H Y Y, Javey A. Wearable sweat sensors[J]. Nature Electronics, 2018, 1(3): 160-171.
[90] Pazos-Outón L M, Szumilo M, Lamboll R, et al. Photon recycling in lead iodide perovskite solar cells[J]. Science, 2016, 351(6280): 1430-1433.
[91] Miller O D, Yablonovitch E, Kurtz S R. Strong internal and external luminescence as solar cells approach the Shockley-queisser limit[J]. IEEE Journal of Photovoltaics, 2012, 2(3): 303-311.
[92] Araujo G L, Marti A. Limiting efficiencies of GaAs solar cells[J]. IEEE Transactions on Electron Devices, 1990, 37(5): 1402-1405.
[93] Gunn J B. Microwave oscillations of current in Ⅲ-V semiconductors[J]. Solid State Communications, 1993, 88(11/12): 883-886.
[94] Roelkens G, Liu L, Liang D, et al. Ⅲ-V/silicon photonics for on-chip and intra-chip optical interconnects[J]. Laser & Photonics Reviews, 2010, 4(6): 751-779.
[95] Sheng X, Yun M H, Zhang C, et al. Device architectures for enhanced photon recycling in thin-film multijunction solar cells[J]. Advanced Energy Materials, 2015, 5(1): 070006.
[96] Ding H, Hong H, Cheng D L, et al. Power- and spectral-dependent photon-recycling effects in a double-junction gallium arsenide photodiode[J]. ACS Photonics, 2019, 6(1): 59-65.
[97] Martı́ A, Balenzategui J L, Reyna R F. Photon recycling and Shockley’s diode equation[J]. Journal of Applied Physics, 1997, 82(8): 4067-4075.
[98] Richter C P. Physiological factors involved in the electrical resistance of the skin[J]. American Journal of Physiology-Legacy Content, 1929, 88(4): 596-615.
Article Outline
盛兴, 赵汶鑫, 李丽珠, 黄云翔, 丁贺. 脑机接口技术的基础研究:神经元与二极管[J]. 中国激光, 2023, 50(9): 0907301. Xing Sheng, Wenxin Zhao, Lizhu Li, Yunxiang Huang, He Ding. Foundation of Brain‐Machine Interfaces: Neurons and Diodes[J]. Chinese Journal of Lasers, 2023, 50(9): 0907301.