光动力疗法产生疼痛的机制以及临床干预手段  下载: 601次
下载: 601次
Photodynamic therapy (PDT) is a new method that uses cytotoxic reactive oxygen species (ROS), which are generated through the interaction of oxygen in tissues and photosensitizers excited by the light of a specific wavelength, to erode the nidus. Currently, PDT has been approved by the Food and Drug Administration (FDA) for the clinical treatment of skin diseases (such as superficial skin cancer, actinic keratosis, squamous cell carcinoma, basal cell carcinoma, port-wine stains, and malignant tumors) and malignant tumors (such as esophageal, gastric, and lung cancers). Compared with traditional treatment methods, PDT has the following advantages: it results in highly selective tissue destruction, is non-invasive, has low side effects, is with no obvious drug resistance, and is easy to combine with other treatment methods.
In the clinical practice of treating skin diseases, patients experience burning, tingling, or pain during treatment with PDT. In contrast, in the treatment of malignant tumors, patients rarely report pain because they are treated with PDT under general anesthesia. The discomfort experienced during PDT often influences the treatment, greatly reducing the efficacy of PDT; some patients even cease the treatment because of the excruciating pain. Figuring out the mechanism of pain during PDT is of great importance because researchers and clinicians can develop targeted drugs and design interventions according to the mechanism.
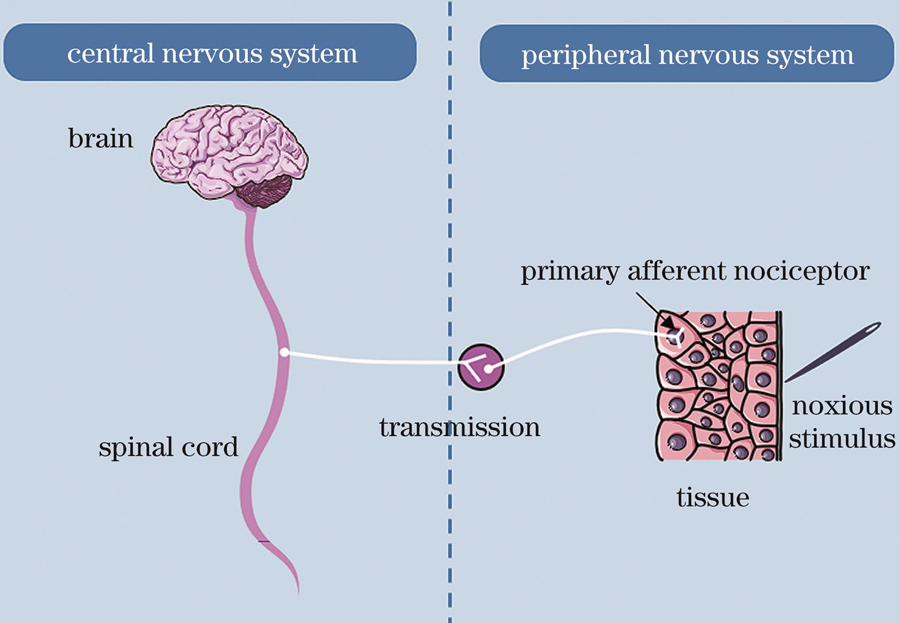
At present, the underlying mechanism of pain during PDT has not been fully elucidated even though the mechanism of pain and changes in the microenvironment caused by PDT have been well studied. In short, the mechanism of pain is explained as follows (Fig. 1). After the tissue is subjected to a specific physical or chemical stimulus, the primary afferent nociceptor converts the painful stimulus into an electrical signal by opening ion channels such as the transient receptor potential vanilloid 1 (TRPV 1) and transient receptor potential ankyrin 1 (TRPA 1). Subsequently, a large influx of calcium ions occurs, the cumulative voltage change results in an action potential in the damaging fibers, and the electrical signals travel from these fibers to the cerebral cortex, where they are perceived as pain. The TRPV 1 and TRPA 1 ion channels are expressed abundantly on the skin surface. When PDT is performed, the light and ROS can stimulate the TRP channels and cause the original potential change and subsequently produce a sense of pain. As for the changes in the microenvironment, an acute inflammation occurs after PDT, and cytokines such as histamine, glutamate, prostaglandin, 5-hydroxytryptamine, substance P, nerve growth factor, and tumor necrosis factor-α are generated, binding to receptors such as α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPA), N-methyl-D-aspartic acid receptor (NMDA), γ-aminobutyric acid (GABA), neurokinin 1 receptor (NK1R), nerve growth factor receptor (NGFR), and tumor necrosis factor receptor (TNFR), which then cause a nerve impulse (Fig. 2).
Various clinical interventions have been applied to manage pain during PDT (Fig. 3), such as drug coating, intravenous injection, inhalation, scalp nerve blocks, treatment site cooling with cold air or water spray, treatment parameter optimization, and sunlight PDT. However, the efficacies of these interventions differ (Table 1): some drug interventions, such as oral drug and facial coating, cause no obvious pain-relieving effect during PDT, while the pain-relieving effect of some other drug interventions, such as intravenous drugs, depends on the treated disease and analgesic type. Unexpectedly, physical interventions (mainly referring to water spray) have a great pain-relieving effect during PDT. This intervention is now chosen by most clinicians because it does not require additional drugs, has minor side effects, and does not influence the therapeutic effect. A reasonable explanation is that by decreasing the temperature of the surface of the skin, the activation threshold of the TRPV 1 channel is lowered, and the original nerve impulses are not easily produced. Therefore, the interventions for targeting the TRP channels are effective to reduce the pain of patients during PDT treatment. Nevertheless, this intervention requires additional instruments, which are not allowed in most hospitals.Therefore, it is promising for researchers or clinicians to develop more targeted drugs or interventions for TRP channels to relieve pain generated during PDT.
In clinical practice, PDT has been widely applied to cure various skin diseases; however, patients always experience excruciating pain and often discontinue the treatment. The main reason for the pain is that PDT has a direct effect on nerve endings by directly stimulating TRP channels. The laser used in PDT directly activates the TRPV1 and TRPA1 channels, causing nerve impulses that eventually send signals to the brain and result in pain. In addition, large amounts of ROS produced locally during PDT can irritate the TRPV1 and TRPA1 channels, causing acute pain. However, at present, there are no clinical intervention measures or drugs targeting the TRPV1 and TRPA1 channels, which are very valuable directions of research on relieving pain generated during PDT.
1 引言
光动力疗法(PDT)是利用特定波长的光照射富集有光敏剂的病灶部位,通过激发光敏剂,将能量转移到组织中的氧气,从而产生具有细胞毒性的活性氧(ROS),最终清除病灶[1]。PDT的三要素是:光敏剂、氧气、光源,自Dougherty等[2]在1975年对小鼠模型注射血卟啉进行PDT,并取得了很好的效果以来,大量研究人员对PDT进行了广泛的研究,目前已经开发出在近红外等皮肤穿透深度更深的光源区域激发的新型光敏剂,并针对病灶部位的乏氧环境提出了PDT结合免疫治疗的新型治疗手段[3-4]。目前,PDT已经被美国食品药品管理局(FDA)批准可用于多种疾病的临床治疗,成为多种皮肤疾病(如浅表性皮肤癌、日光性角化病、鳞状细胞癌、基底细胞癌、鲜红斑痣等)和恶性肿瘤(如食管癌、胃癌、肺癌等)的有效治疗手段。5-氨基乙酰丙酸(ALA)和5-氨基乙酰丙酸甲酯(MAL)是皮肤科临床中常使用的两种光敏剂[5]。将ALA和MAL涂敷在病灶部位表面,其经过角质细胞吸收进入组织,通过血红素合成途径发生累积,在累积了足够量的内源性光敏剂原卟啉Ⅸ(PpⅨ)后,可在激光激活下产生光动力效应[6-7]。
与传统治疗手段相比,PDT具有以下优势:组织高度选择性破坏、无创、低副作用、无明显耐药性、容易与其他治疗手段结合[8]。在皮肤疾病治疗的临床实践中,患者在治疗过程中会有灼烧感、刺痛或疼痛感。但在治疗恶性肿瘤的研究中却鲜有关于患者疼痛的报道,这是由于恶性肿瘤患者是在全麻状态下接受PDT治疗,并没有感知疼痛的能力。虽然皮肤病患者在接受PDT治疗时痛感会因个人体质而有所差异,但是这些不适感在很大程度上影响了患者进行PDT治疗的过程。目前PDT产生疼痛的相关机理并没有被完全阐明。有学者研究了PDT治疗过程中瞬时受体电位(TRP)离子通道的情况,如瞬时受体电位通道香草酸亚型1(TRPV1)和瞬时受体电位锚蛋白1(TRPA1),证明在PDT治疗过程中TRPV1和TRPA1通道会被激活[9]。在TRP通道被激活后,出现钙离子大量内流,导致神经细胞产生电位变化,最终信号被传递给大脑,个体产生疼痛感。而且PDT治疗过程伴随着细胞的多种死亡机制,机体会发生急性炎症以清除坏死组织,保证内环境的稳定性,也会导致患者急性疼痛[10]。ROS会直接刺激神经细胞的细胞膜表面TRPV1通道,产生神经冲动,这是PDT产生疼痛的一个重要因素[9,11-12]。此外,有学者证明激光也可以直接刺激TRPV1通道和TRPA1通道,进而造成痛感的产生[9,13]。
目前临床中已经从多种角度采取各种措施来缓解治疗过程中的相关疼痛,包括:术前在患者面部涂敷利多卡因凝胶、口服曲马多;术中利用冷风机对治疗部位进行冷喷;术后静脉注射凯纷;进行全身麻醉等。靳峰阳等[14]从光敏剂类型及浓度、皮损部位及类型、光辐照强度、光源类型和辐照波长等角度,研究了PDT中疼痛的相关因素,为临床医生提供了有价值的参考。
本文对相关PDT中疼痛的机理进行了整理,并对报道过的临床干预方法进行总结,旨在使临床医生能更好地理解PDT中的疼痛机理,并为以后的临床治疗与相关研究提供指导。
2 疼痛
2.1 产生疼痛的机理
所有的感官系统都必须将环境刺激转换为电化学信号。在视觉或者嗅觉中,初级感觉神经元只需要检测一种类型的刺激(光或者气味),然而由于具有疼痛感知能力的初级感觉神经元能检测多种刺激方式,疼痛产生的机理与别的感受相比更加复杂[15]。
2.2 TRP通道
瞬时受体电位通道是一系列细胞的环境信号传感器,目前受到学者的极大关注[20]。而TRP通道的一个亚型——TRPV1蛋白的发现使人们在分子层面对疼痛有了更好的理解[21]。TRPV1通道能够被外界有害环境刺激,比如高温(>43 ℃)[21-22]、酸性环境(pH<5.9)[23],也能被一些有刺激性的物质激活,比如辣椒素[21]、樟脑[24]、胡椒粉[25]等。另外,一些炎性因子,如细胞因子、前列腺素、缓激肽、谷氨酸、5-羟色胺、神经生长因子等也能刺激TRPV1通道[26-28],使体内的炎症环境可以被检测到。皮肤中的初级传入感觉纤维和非神经元细胞(角质细胞、肥大细胞、树突状细胞、T细胞、内皮细胞等)均有TRPV1的表达[29-31],因此TRPV1通道对皮肤中的免疫调节也有非常关键的作用[32]。TRPV1激活会引起持续性的膜去极化、激发动作电位和增加突触活动,将电信号传导至中枢神经系统,使人体感受到疼痛[33]。
TRPA1是TRP通道的另一个亚型,在化学性疼痛和机械性疼痛的产生过程中都起到一定的作用[34]。TRPA1常见的刺激性物质有:芥末(有效成分为异硫氰酸丙烯酯)和大蒜(有效成分为大蒜素),另外一些环境污染烟雾(比如汽车尾气、植物燃烧产生的烟雾等)、催泪瓦斯等同样能够通过激活TRPA1通道刺激眼睛和呼吸系统的神经元,造成疼痛和炎症的发生[35-37]。值得注意的是,ROS也能激活TRPA1通道[9,38-39],这是PDT中产生疼痛的原因之一。
在皮肤伤害神经末梢有高度表达的TRPV1和TRPA1通道[40],PDT治疗皮肤病产生的疼痛与上述两个通路有很大的关系。下文我们将讨论TRPV1通路和TRPA1通路与PDT产生疼痛的关系。
3 PDT与疼痛
在临床治疗过程中,光敏剂的给药方式有“局部涂敷”和“静脉注射”两种方式,然而在治疗过程中,接受两种给药方式的患者均会有不同程度的疼痛感,这证明PDT产生的疼痛与给药方式无关。我们从PDT造成的微环境变化与疼痛产生的机制两个角度,论述了PDT造成疼痛的潜在机制。在PDT治疗过程中,体内微环境会发生一系列的变化,除了光敏剂被激发后会直接产生ROS外,体内免疫系统也会被激活,并与神经系统相互作用,产生的神经冲动最终被传递到大脑,使人体感受到疼痛(
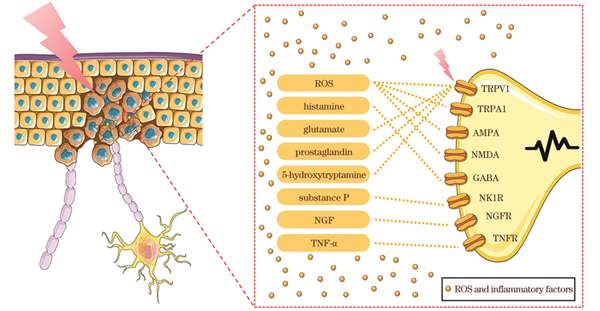
图 2. PDT治疗过程中疼痛产生的潜在机制
Fig. 2. Potential mechanisms of pain production during PDT therapy
3.1 PDT产生的ROS诱导疼痛
已有的研究表明,PpⅨ荧光与PDT治疗期间的疼痛体验呈正相关,而ROS作为PpⅨ的代谢产物和PDT的效应物,又会参与到神经性疼痛和辣椒素诱导的疼痛之中。ROS可以促进N-甲基-D-天冬氨酸受体(NMDA受体)和α-氨基-3-羟基-5-甲基-4-异恶唑丙酸受体(AMPA受体)的磷酸化[41],并减少γ-氨基丁酸(GABA)的释放,从而诱导伤害性感受神经元放电或使其放电率增加[42-43]。
有研究认为,ROS的量与PDT治疗期间的疼痛及疗效有关,ROS局部浓度的降低会减少神经末梢的刺激,缓解PDT治疗期间的疼痛强度[44],而ROS的总量可以决定PDT的疗效。临床上可以在PDT中通过减少ROS的量来减弱患者在治疗期间的疼痛感,通过降低温度或减少光通量来降低PpⅨ的转化率以及ROS的累积量[45-46]。这些改进方法与正常剂量相比,在相同的治疗时间条件下虽然可以减少疼痛,但也会导致临床治愈率降低。在这种情况下通过延长治疗时间,光动力疗法的临床治愈率可以与普通PDT接近。
3.2 PDT造成的局部炎症诱导疼痛
3.2.1 PDT造成的局部炎症
在PDT中,对细胞产生直接毒性并导致细胞死亡的是光敏剂激活后与氧气反应生成的ROS。短时间内产生大量ROS,会造成体内氧化应激,释放肿瘤坏死因子α(TNF-α)、一氧化氮(NO)、组胺(histamine)、前列腺素E2(prostaglandin E2)和其他细胞因子,导致炎症反应发生[47]。在PDT治疗后,经常会出现组织水肿的现象,这可能是生成的伤害性神经肽——P物质(substance P)导致的[34]。
在PDT治疗过程中,细胞被杀死,病灶部位组织被破坏,机体为了防止碎片扩散,及时清除碎片,恢复组织的正常功能,保持内环境的稳定,这样会引发局部炎症反应。在动物模型与临床实践中,PDT的效果在一定程度上还取决于免疫系统的完整性[9]。光敏剂被激发后产生ROS,对肿瘤组织造成了创伤性损伤,引起氧化应激,体内免疫系统出现急性炎症以清除组织碎片,之后恢复体内平衡[48]。目前,已经有多项研究表明,PDT诱导的急性炎症能够增强全身的抗肿瘤免疫力[49-51]。
对于PDT治疗肿瘤,肿瘤组织被破坏,导致花生四烯酸代谢产物(如前列腺素、白三烯和血栓素)释放、炎症因子[如趋化因子CXC配体2(CXCL2)、白介素6(IL-6)、白介素1β(IL-1β)、TNF-α]的水平上调以及补体的激活[9]。这些因素共同作用,使得先天免疫细胞浸入肿瘤组织,有利于攻击和清除死亡的肿瘤细胞。此外,PDT在局部或全身诱导补体激活,随后肿瘤组织释放过敏性毒素,使得血管通透性增加[52],导致中性粒细胞在光损伤的肿瘤组织的血管界面上聚集,血管壁内外出现梯度差,促进中性粒细胞向肿瘤内部浸润。随后,中性粒细胞通过白介素17(IL-17)和IL-1β驱动的巨噬细胞炎症蛋白2(MIP2)介导的途径,经高内皮微静脉进入肿瘤引流淋巴结(TDLN)[53],激活抗肿瘤CD8+细胞,进而提高抗肿瘤的疗效。PDT会引导肿瘤细胞释放热休克蛋白70(HSP70),最后导致巨噬细胞经过Toll样受体(TLR)后被激活为M1型巨噬细胞,并释放TNF-α[54]。被激活的巨噬细胞还会分泌IL-1β和神经生长因子(NGF),这些细胞因子会在PDT治疗后对肿瘤细胞产生持续的杀伤作用,有助于增强PDT的治疗效果,促进组织损伤后的恢复。
根据研究,PDT过程产生的局部炎症是引起慢性疼痛的主要原因。在炎症性疼痛中,痛觉感受器神经元-免疫系统的相互作用起着关键作用,免疫和伤害性感受系统可以识别破坏性和有害性刺激,从而驱动反应,防止组织损伤,并恢复稳态,这些相互作用的失调是皮肤、关节、呼吸道和胃肠道炎症性疾病的发病机制的基础[34]。
3.2.2 炎性因子刺激TRPV1和TRPA1
PDT造成急性炎症后,机体释放的各种炎症介质会刺激感觉神经元。这些炎症介质通过激活多种蛋白激酶来增强TRPV1通道的磷酸化,并可作为TRPV1通道的内源性配体发挥作用。
白介素1(IL-1)是一种有效的促炎因子,与神经病理性疼痛有关。Reeve等[55]在健康大鼠鞘内注射IL-1,发现大鼠出现神经病理性疼痛的症状。Jin等[56-57]发现,将人的外成膜纤维细胞与IL-1β一起培养,能显著提高白介素8(IL-8)和IL-6的水平,并上调炎症分子(如诱导型NO、前列腺素E2和TNF-α等)。而NO和前列腺素都是痛觉过敏的重要介质,尤其在炎症组织[58-60]。此外,IL-6与其受体结合后可以反过来促进IL-1β诱导糖蛋白代谢,IL-1β和IL-6之间存在正反馈循环[61]。因此,IL-1β可以作为炎症级联放大的启动条件。
PDT治疗过程中巨噬细胞被激活而释放的TNF能够增强机体对细胞的杀伤效果,且TNF的表达水平与痛觉超敏或痛觉过敏的发展存在相关性[62-63]。Bennett[64]利用由TNF-α或环孢素A合成的特异性抑制剂能够显著减少神经病理性疼痛的反应。组织炎症造成的神经损伤能够增加TNF的受体(TNFR)——TNFR1和TNFR2的表达[65],这是TNF能够造成疼痛过敏的原因[66]。
肥大细胞会聚集在PDT产生炎症损伤的部位,释放NGF、组胺等物质,并与其受体即神经生长因子受体(NGFR)、GABA结合,引起痛觉过敏。NGF与络氨酸激酶受体(trkA)结合后,会导致TRPV1受体的磷酸化和致敏,这是NGF诱导热痛觉过敏的原因[67]。另外,NGF会在长时间内造成伤害感受器的敏感性增加,这是由于在NGF-trkA逆运输至细胞核后,影响了TRPV1、电压门控钠离子通道的亚型Nav1.8、脑源性神经营养因子(BNDF)和P物质等伤害感受器表达的调节[68]。肥大细胞迁移到炎症组织,随后释放组胺,引起痛觉过敏。Kajihara等[69]在体外培养小鼠背根神经节(DRG)细胞时发现,组胺能够刺激TRPV1通道介导的Ca2+内流,通过H1受体激活TRPV1通道并与磷脂酶C(PLC)/蛋白激酶C(PKC)途径结合,导致机体对疼痛的反应敏感。
P物质在初级感觉神经元中有表达,在感受伤害性刺激的过程中有着重要作用。当P物质与神经激肽1受体(NK1R)结合后,从内体发出信号,诱导神经元的持续兴奋和疼痛传递[70-71]。
3.3 光刺激神经系统诱导疼痛
光敏化是一种对无害光过度敏感的现象。Babes等[9]发现,卟啉病患者皮肤暴露在405 nm光下会出现中度疼痛,这是由于蓝光能够显著激活TRPA1通道,并轻度激活TRPV1通道。另外,蓝光(470~480 nm)直接刺激角质细胞中的TRPV1通道,导致NF-κB和AP-1信号通路被激活,最终造成促炎因子的分泌[13]。Negri等[72]利用激光刺激含有TRPV1的内皮集落形成细胞,并用TRPV1的抑制剂——辣椒平作对照实验,发现在不加入辣椒平的实验组中,其钙离子内流现象明显,证明了光可直接刺激TRPV1通道,进而导致钙离子内流。
综上所述,PDT产生具有细胞毒性的ROS后,会引起免疫系统对坏死部位进行一系列的免疫调节,产生急性炎症。这些炎症反应一方面有助于机体修复破坏组织、清除组织碎片、恢复体内平衡[10];另一方面,在急性炎症过程中,免疫细胞释放各种能够调节伤害感受器神经活动的疼痛敏感性介质,导致疼痛感的产生[34]。然而在患者治疗过程中,急性疼痛往往会影响正常的治疗过程。
针对PDT治疗过程中产生的疼痛,研究者们采取了一系列的措施,以期缓解或者能够完全抑制疼痛。
4 干预措施
疼痛是一种非常个性化的主观体验,是一种涉及感官和情感特征的多维体验,临床上对患者遭受的疼痛一般采用问询的方式进行评分。临床治疗中疼痛的评价系统有以下几种:数字评分量表(NRS,0~10分)、疼痛强度数字评分表(PI-NRS,0~10分)、视觉模拟量表(VAS,0~10分)和面部疼痛量表(FPS,0~6分)[73]。每种量表中评分数字从小到大表示患者疼痛程度加深,评分为0时表示患者没有疼痛感,评分最高时认为患者遭受了想象中最疼痛的感觉。需要注意的是,在一些研究中,VAS的范围为0~100。目前临床中对PDT治疗过程中产生的疼痛采取了一些缓解措施。根据干预措施的使用手段进行分类,可以分为药物干预、物理干预以及调整治疗参数。
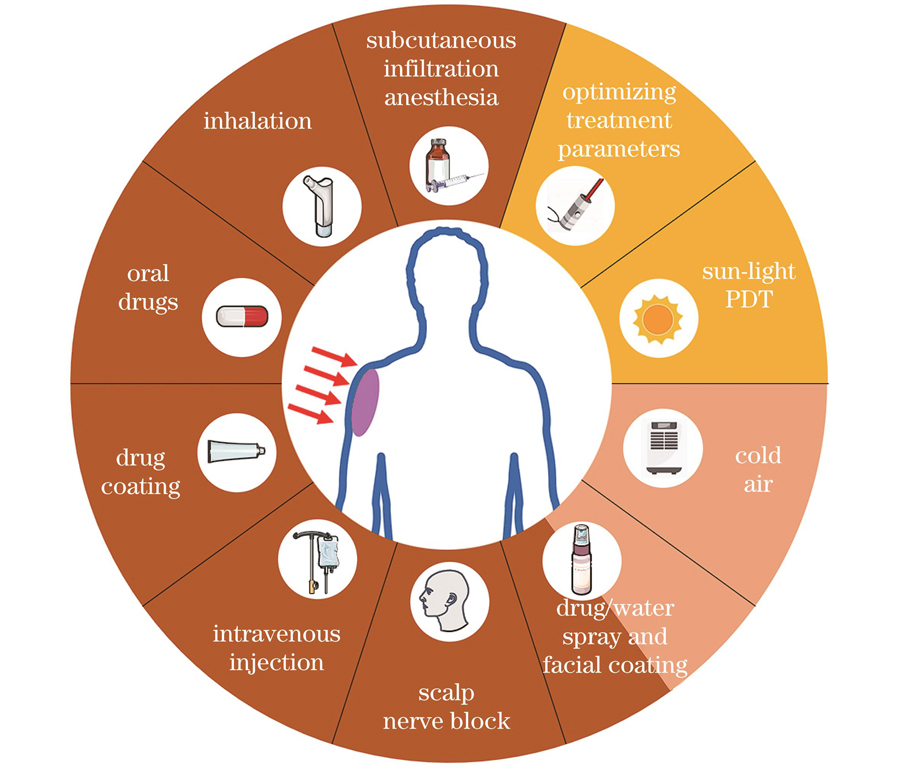
图 3. 临床缓解PDT治疗过程中产生的疼痛的干预手段
Fig. 3. Clinical interventions to relieve pain during PDT treatment
4.1 药物干预
4.1.1 口服镇痛药物
Hambly等[74]总结了200例日光性角化病、鲍恩病和浅表性基底细胞癌在PDT治疗过程中产生的疼痛情况。在这些病例中,有20%的患者在治疗前30 min服用止痛药,其中服用扑热息痛的患者占88%,服用氨酚待因的患者占10%,服用布洛芬的患者占2%。未服用止痛药物患者的平均VAS值为37.8,而服用止痛药患者的平均VAS值为56.6,证明服用止痛药并不会缓解PDT治疗过程中产生的疼痛。Miller等[75]也发现,服用止痛药患者的疼痛评分明显高于未服用止痛药的患者,说明服用止痛药对缓解PDT过程中的疼痛并没有益处。
4.1.2 静脉注射镇痛药物
氟比洛芬酯能够有效减轻外周及中枢神经敏感化,能够选择性地在炎症以及血管损伤部位聚集,目前已经被广泛应用在外科手术中。周俊等[76]利用海姆泊芬对鲜红斑痣患者进行治疗,在治疗前采用静脉滴注氟比洛芬酯的方式来缓解患者治疗过程中的疼痛感,结果证明这种方法有效缓解了治疗过程中产生的疼痛。
4.1.3 皮下浸润麻醉
Borelli等[77]采用皮下浸润麻醉的手段干预ALA-PDT过程,发现皮下浸润麻醉能够有效缓解患者在治疗过程中的疼痛感,而且干预手段并不会影响临床效果。虽然注射麻醉剂有用,但是在大面积的疼痛易感区域进行皮下浸润麻醉,会导致相当大的疼痛,引起患者的焦虑心理[78]。
4.1.4 头皮神经阻滞
Klein等[79]利用MAL-PDT治疗日光性角化病患者,在治疗前30~40 min时间内,采用头皮神经阻滞的方式缓解疼痛,并将该方法与静脉注射哌腈米特(7.5 mg,治疗前30 min注射)、口服安乃近联合冷风镇痛两种干涉策略进行对比。研究发现,相较于另外两种干预措施,头皮神经阻滞方法有效缓解了患者在PDT治疗过程中的疼痛感,是一种有效的缓解疼痛的干预措施。
4.1.5 面部涂敷或者喷雾
Skiveren等[80]对28名日光性角化病和基底细胞癌患者进行PDT治疗,手术前在治疗部位预先用质量分数为0.3%的吗啡凝胶进行处理,以期缓解患者在治疗过程中的疼痛感。实验发现,外用吗啡凝胶对缓解PDT治疗过程中的疼痛无效。Liu等[81]在利用海姆泊芬光动力疗法治疗鲜红斑痣时,采用外涂利多卡因乳膏作为干预措施来缓解治疗过程中的疼痛感。他们在临床实践中发现,在病变部位、大小、类型等各方面,与对照组相比,使用利多卡因乳膏组的疼痛评分并无显著性差异,使用利多卡因乳膏并不能缓解治疗期间的疼痛感。这是利多卡因在皮肤中的渗透能力不强导致的。Holmes等[82]在对浅表基底细胞癌、鲍恩病或日光性角化病等进行ALA-PDT治疗时,采用表面涂敷透皮性能比利多卡因乳膏更强的丁卡因凝胶作为缓解疼痛的干预手段,发现涂敷丁卡因凝胶也不会缓解患者的不适感,这可能是ALA使皮肤组织饱和、限制丁卡因渗透造成的。另外,有研究表明,利多卡因、可卡因、普鲁卡因等碱性药物由于其高pH值会导致光敏剂失去化学活性[80]。
Anseline等[83]在ALA-PDT治疗日光性角化病的过程中,使用一种有效成分为洋甘菊和薄荷醇等物质的喷雾进行干预,缓解了患者的疼痛。喷雾有效成分对TRPA1、TRPA1、TRMP8等离子通道起到阻断作用,术前的预处理、喷雾剂的冷却作用也会对缓解相关疼痛起到一定的作用。然而,喷雾干预手段与冷喷镇痛作用类似,其有效成分是否真正发挥了作用还需要进一步研究。
4.1.6 吸入气体镇痛
由N2O(医用笑气,体积分数为50%)和O2(医用氧气,体积分数为50%)组成的混合气体可用来治疗短期疼痛,且一旦停止吸气,气体会通过肺部迅速排泄到体外,患者快速恢复正常状态。Cabete等[84]利用这种混合气体辅助光动力疗法治疗外阴硬化性苔藓,有效缓解了患者在治疗过程中遭受的疼痛,而且在患者不失去知觉的前提下,有抗焦虑和镇静作用。Fink等[85]也利用这种混合气体来缓解日光性角化症患者在PDT治疗过程中的疼痛感,总体疼痛减轻了55.2%。
药物干预是一种常用的临床镇痛手段。口服药物的干预方式虽然简单,但是对于缓解PDT治疗过程中的疼痛并没有益处。静脉注射、皮下浸润麻醉以及头皮神经阻滞等手段可以有效缓解患者在治疗过程中的疼痛,但是均须采用额外的临床手段,对患者而言有一定的风险性,而且在药物注射过程中会出现不同程度的疼痛,给部分患者带来术前心理阴影。面部涂敷或者喷雾等手段也是一种简单的术前干预手段,但是药物的透皮性能对疼痛缓解的效果起到了决定性的作用。而吸入气体镇痛方式的缓解时长较短,在长时间PDT治疗过程中需要多次吸入,影响治疗效果。因此,基于现有临床PDT治疗过程,采用一种无需额外操作的给药方式将镇痛药物递送至病灶部位是很有意义的。然而,镇痛药物与光敏剂之间的相互作用以及两种药物的起效时间需要进一步研究探讨。
4.2 物理干预
临床中采用的物理干预手段主要是冷喷方式。皮肤伤害神经末梢有高度表达的TRPV1,采用物理干预手段,主要目的是降低治疗部位温度,进而降低TRPV1通道的激活阈值,最终达到缓解疼痛的效果。冷喷装置易于应用,而且目前已经有大量临床数据证明,采用冷喷装置并不会影响PDT的治疗效果。因此,临床中倾向于选择冷喷装置(喷水器或者空气冷却装置)来缓解治疗过程中的疼痛感[86-88]。Wiegell等[45]在利用MAL-PDT治疗日光性角化病的过程中,采用冷喷水雾(水雾温度为5 °C)和表面覆盖“冷却包”的方法来缓解患者在治疗过程中的疼痛感,但是这两种方法都只能缓解一小部分的疼痛。虽然治疗过程中的冷却措施能够使治疗更具有耐受性,但这与疼痛减轻程度关系不大,更多的是试图减轻疼痛的心态以及冷却过程中护士一直在现场带来的镇静效果,以上因素缓解了患者对疼痛的感知。
虽然冷喷装置能够很好地缓解患者在PDT治疗过程中遭受的疼痛感,但是冷喷装置往往需要额外的设备和场地,这给治疗过程带来额外的负担。因此,对现有光动力疗法的操作过程进行改良是十分必要的。
4.3 调整治疗参数
4.3.1 激光波长对疼痛的影响
Osiecka等[89]利用ALA-PDT对日光性角化病进行红光和绿光的治疗,每隔两周进行3次光动力治疗,经过9个月的观察,发现红光和绿光的治疗效果没有显著差异(红光和绿光的治愈效果分别为91.67%和86.67%),然而红光照射区域的平均疼痛值明显高于绿光。这主要是由于采用红光对皮肤进行治疗时,皮肤会产生热量,进而激活TRPV1通道,出现痛感。
4.3.2 激光辐照度对疼痛的影响
高光剂量率往往会使光敏剂在短时间内被漂白,使局部氧气耗尽,最终导致动力效率的丧失,而且会增加治疗期间的疼痛感[90]。改变光源辐照度可以通过降低光源能量、增加光源距离、延长曝光时间等手段来实现。辐照度与光源和治疗区域之间的距离成反比。在治疗过程中,当光源与皮肤之间的距离从8 cm增加到25 cm时,光源辐照度下降了一半,患者在治疗过程中的疼痛感也降低了[91]。但Ericson等[46]利用ALA-PDT治疗日光性角化病时,使用辐照度为30~75 mW/cm2的光源,发现疼痛在治疗开始时最剧烈,随后趋于平稳,这一事实可能与光敏剂水平有关,光敏剂在治疗开始时水平较高。随着PpⅨ浓度的降低,治疗过程会减慢,从而疼痛感减轻。这一论点暗示疼痛对光动力过程有时间和剂量依赖性,但不能排除伤害感受器的脱敏和/或患者对治疗的适应导致了疼痛减轻。另外,低剂量LED光源在临床中也会有很好的治疗效果[92]。
Gholam等[93]使用ALA-PDT治疗日光性角化病时发现,与传统PDT相比,低辐照度PDT(激光辐照度比传统PDT中使用的激光低25%)下患者在治疗过程中的中断次数显著减少,而且治疗效果相当。Cottrell等[94]在利用ALA-PDT治疗浅表性基底细胞癌的过程中,先采用低辐照度激光对病灶部位进行治疗,在光敏剂被漂白90%之后,换用高辐照度的激光进行连续照射,直至光剂量达到200 J/cm2,这种方案的临床疗效与连续使用高光剂量率的激光进行连续治疗相当,但是患者的疼痛感却有明显的缓解。
4.3.3 日光‑PDT
日光-PDT已被广泛用于治疗日光性角化病,治疗过程中的疼痛感和炎症非常轻[95]。Nguyen等[96]综述了与日光-PDT相关的研究,总结出2 h的日光照射足以保证治疗完成,而且疗效似乎不受天气条件的限制,适用于疼痛耐受度低或计划不可用的患者。然而,由于地理位置不同,各地阳光辐照强度也不尽相同,而且日光-PDT有明显的季节依赖性,因此需要进一步研究日光-PDT,确定不同地理位置处日光-PDT具有最佳疗效的时间[97-98]。
表 1. PDT治疗过程中缓解疼痛的干预手段以及效果
Table 1. Intervention means and effects of relieving pain during PDT treatment
|
5 结束语
在临床实践中,PDT已被广泛用于治疗各种皮肤疾病。然而,在治疗过程中,患者往往会感受到难以忍受的疼痛,造成治疗过程中断,甚至终止治疗,极大地削弱了光动力疗法的疗效。临床进行PDT治疗前会根据患者的年龄、病灶部位、疾病严重程度等进行评估,然后再制定特定的方案,因此利用临床数据对疼痛进行分析无法标准化。而且,不同部位皮肤的神经细胞含量不同,不同部位的疾病区域在治疗时也会出现明显的疼痛差异。另外,疼痛还依赖于患者的主观感受,每个人的耐受性不同,心理作用对患者感知疼痛也有明显的影响。最重要的是,疼痛对于人体还有警示和保护作用,将疼痛降至最低对于人体而言是有潜在危险的。因此,没有一个适用于所有患者的衡量疼痛的标准,开发疼痛的标准衡量模式极为困难。
光动力疗法对神经系统末梢有直接影响,尤其是可直接刺激TRP通道。PDT治疗过程中使用的激光会直接激活TRPV1和TRPA1通道,造成神经冲动,最终将信号传递到大脑,使人感受到疼痛。另外,PDT治疗过程中在局部产生的大量ROS会刺激TRPV1和TRPA1通道,造成急性疼痛。然而,目前并没有针对TRPV1和TRPA1通道的临床干预措施,这是一个极有价值的研究方向。
从光动力刺激细胞死亡的原理出发,并结合皮肤表面产生疼痛的机制,阐述了光动力疗法造成疼痛的潜在机理,还对已经报道过的临床干预手段进行了总结,为后续的相关研究提供了参考。
[1] Lucky S S, Soo K C, Zhang Y. Nanoparticles in photodynamic therapy[J]. Chemical Reviews, 2015, 115(4): 1990-2042.
[2] Dougherty T J, Grindey G B, Fiel R, et al. Photoradiation therapy. Ⅱ. cure of animal tumors with hematoporphyrin and light[J]. JNCI: Journal of the National Cancer Institute, 1975, 55(1): 115-121.
[3] Chilakamarthi U, Giribabu L. Photodynamic therapy: past, present and future[J]. Chemical Record, 2017, 17(8): 775-802.
[4] Du B J, Tung C H. Enzyme-assisted photodynamic therapy based on nanomaterials[J]. ACS Biomaterials Science & Engineering, 2020, 6(5): 2506-2517.
[5] Griffin L L, Lear J T. Photodynamic therapy and non-melanoma skin cancer[J]. Cancers, 2016, 8(10): 98.
[6] Kennedy J C, Marcus S L, Pottier R H. Photodynamic Therapy (PDT) and Photodiagnosis (PD) using endogenous photosensitization induced by 5-Aminolevulinic Acid (ALA): mechanisms and clinical results[J]. Journal of Clinical Laser Medicine & Surgery, 1996, 14(5): 289-304.
[7] Choi S H, Kim K H, Song K H. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial[J]. Journal of the European Academy of Dermatology and Venereology: JEADV, 2015, 29(8): 1598-1605.
[8] Lan M H, Zhao S J, Liu W M, et al. Photosensitizers for photodynamic therapy[J]. Advanced Healthcare Materials, 2019, 8(13): e1900132.
[9] Babes A, Sauer S K, Moparthi L, et al. Photosensitization in porphyrias and photodynamic therapy involves TRPA1 and TRPV1[J]. The Journal of Neuroscience, 2016, 36(19): 5264-5278.
[10] Falk-Mahapatra R, Gollnick S O. Photodynamic therapy and immunity: an update[J]. Photochemistry and Photobiology, 2020, 96(3): 550-559.
[11] Gwak Y S, Hassler S E, Hulsebosch C E. Reactive oxygen species contribute to neuropathic pain and locomotor dysfunction via activation of CamKII in remote segments following spinal cord contusion injury in rats[J]. Pain, 2013, 154(9): 1699-1708.
[12] Wright L, Baptista-Hon D, Bull F, et al. Menthol reduces phototoxicity pain in a mouse model of photodynamic therapy[J]. Pain, 2018, 159(2): 284-297.
[13] Yoo J A, Yu E, Park S H, et al. Blue light irradiation induces human keratinocyte cell damage via transient receptor potential vanilloid 1 (TRPV1) regulation[J]. Oxidative Medicine and Cellular Longevity, 2020, 2020: 8871745.
[14] 靳峰阳, 石卫东. 光动力疗法相关性疼痛治疗的新进展[J]. 中国皮肤性病学杂志, 2019, 33(12): 1429-1432.
Jin F Y, Shi W D. New advances in the management of photodynamic therapy-associated pain[J]. The Chinese Journal of Dermatovenereology, 2019, 33(12): 1429-1432.
[15] Julius D, Basbaum A I. Molecular mechanisms of nociception[J]. Nature, 2001, 413(6852): 203-210.
[16] Stucky C L, Gold M S, Zhang X. Mechanisms of pain[J]. Proceedings of the National Academy of Sciences of the United States of America, 2001, 98(21): 11845-11846.
[17] Julius D. TRP channels and pain[J]. Annual Review of Cell and Developmental Biology, 2013, 29: 355-384.
[18] Burgess P R, Perl E R. Myelinated afferent fibres responding specifically to noxious stimulation of the skin[J]. The Journal of Physiology, 1967, 190(3): 541-562.
[19] Woolf C J, Salter M W. Neuronal plasticity: increasing the gain in pain[J]. Science, 2000, 288(5472): 1765-1769.
[20] Liao M F, Cao E H, Julius D, et al. Structure of the TRPV1 ion channel determined by electron cryo-microscopy[J]. Nature, 2013, 504(7478): 107-112.
[21] Caterina M J, Schumacher M A, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway[J]. Nature, 1997, 389(6653): 816-824.
[22] Vandewauw I, De Clercq K, Mulier M, et al. A TRP channel trio mediates acute noxious heat sensing[J]. Nature, 2018, 555(7698): 662-666.
[23] Tominaga M, Caterina M J, Malmberg A B, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli[J]. Neuron, 1998, 21(3): 531-543.
[24] Xu H X, Blair N T, Clapham D E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism[J]. The Journal of Neuroscience, 2005, 25(39): 8924-8937.
[25] Szallasi A, Blumberg P M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper[J]. Neuroscience, 1989, 30(2): 515-520.
[26] Sikand P, Premkumar L S. Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse[J]. The Journal of Physiology, 2007, 581(2): 631-647.
[27] Sugiura T, Tominaga M, Katsuya H, et al. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1[J]. Journal of Neurophysiology, 2002, 88(1): 544-548.
[28] Zhang X M, Huang J H, McNaughton P A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels[J]. The EMBO Journal, 2005, 24(24): 4211-4223.
[29] Denda M, Fuziwara S, Inoue K, et al. Immunoreactivity of VR1 on epidermal keratinocyte of human skin[J]. Biochemical and Biophysical Research Communications, 2001, 285(5): 1250-1252.
[30] Gouin O, L’Herondelle K, Lebonvallet N, et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization[J]. Protein & Cell, 2017, 8(9): 644-661.
[31] Luo Y H, Suttle A, Zhang Q J, et al. Transient receptor potential (TRP) ion channels in orofacial pain[J]. Molecular Neurobiology, 2021, 58(6): 2836-2850.
[32] Fernandes E S, Fernandes M A, Keeble J E. The functions of TRPA1 and TRPV1: moving away from sensory nerves[J]. British Journal of Pharmacology, 2012, 166(2): 510-521.
[33] Carbone E. Noradrenergic inhibition of presynaptic TRPV1 channels: a new pathway of pain control[J]. The Journal of Physiology, 2017, 595(8): 2413-2414.
[34] Pinho-Ribeiro F A, Verri W A,, Chiu I M. Nociceptor sensory neuron-immune interactions in pain and inflammation[J]. Trends in Immunology, 2017, 38(1): 5-19.
[35] Bautista D M, Jordt S E, Nikai T, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents[J]. Cell, 2006, 124(6): 1269-1282.
[36] Bessac B F, Jordt S E. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control[J]. Physiology, 2008, 23: 360-370.
[37] Caceres A I, Brackmann M, Elia M D, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(22): 9099-9104.
[38] Sawada Y, Hosokawa H, Matsumura K, et al. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide[J]. The European Journal of Neuroscience, 2008, 27(5): 1131-1142.
[39] Hill K, Schaefer M. Ultraviolet light and photosensitising agents activate TRPA1 via generation of oxidative stress[J]. Cell Calcium, 2009, 45(2): 155-164.
[40] Ichikawa H, Sugimoto T. VR1-immunoreactive primary sensory neurons in the rat trigeminal ganglion[J]. Brain Research, 2001, 890(1): 184-188.
[41] Gao X, Kim H K, Chung J M, et al. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain[J]. Pain, 2007, 131(3): 262-271.
[42] Yowtak J, Lee K Y, Kim H Y, et al. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release[J]. Pain, 2011, 152(4): 844-852.
[43] Lee D Z, Chung J M, Chung K, et al. Reactive oxygen species (ROS) modulate AMPA receptor phosphorylation and cell-surface localization in concert with pain-related behavior[J]. Pain, 2012, 153(9): 1905-1915.
[44] Borgia F, Giuffrida R, Caradonna E, et al. Early and late onset side effects of photodynamic therapy[J]. Biomedicines, 2018, 6(1): 12.
[45] Wiegell S R, Haedersdal M, Wulf H C. Cold water and pauses in illumination reduces pain during photodynamic therapy: a randomized clinical study[J]. Acta Dermato-Venereologica, 2009, 89(2): 145-149.
[46] Ericson M B, Sandberg C, Stenquist B, et al. Photodynamic therapy of actinic keratosis at varying fluence rates: assessment of photobleaching, pain and primary clinical outcome[J]. British Journal of Dermatology, 2004, 151(6): 1204-1212.
[47] Ibbotson S H, Wong T H, Morton C A, et al. Adverse effects of topical photodynamic therapy: a consensus review and approach to management[J]. British Journal of Dermatology, 2019, 180(4): 715-729.
[48] Alzeibak R, Mishchenko T A, Shilyagina N Y, et al. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future[J]. Journal for Immunotherapy of Cancer, 2021, 9(1): e001926.
[49] Cecic I, Korbelik M. Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors[J]. Cancer Letters, 2002, 183(1): 43-51.
[50] Dougherty T J, Gomer C J, Henderson B W, et al. Photodynamic therapy[J]. JNCI: Journal of the National Cancer Institute, 1998, 90(12): 889-905.
[51] Gollnick S O, Evans S S, Baumann H, et al. Role of cytokines in photodynamic therapy-induced local and systemic inflammation[J]. British Journal of Cancer, 2003, 88(11): 1772-1779.
[52] Cecic I, Sun J H, Korbelik M. Role of complement anaphylatoxin C3a in photodynamic therapy-elicited engagement of host neutrophils and other immune cells[J]. Photochemistry and Photobiology, 2006, 82(2): 558-562.
[53] Brackett C M, Muhitch J B, Evans S S, et al. IL-17 promotes neutrophil entry into tumor-draining lymph nodes following induction of sterile inflammation[J]. Journal of Immunology, 2013, 191(8): 4348-4357.
[54] Korbelik M, Sun J H, Cecic I. Photodynamic therapy–induced cell surface expression and release of heat shock proteins: relevance for tumor response[J]. Cancer Research, 2005, 65(3): 1018-1026.
[55] Reeve A J, Patel S, Fox A, et al. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat[J]. European Journal of Pain, 2000, 4(3): 247-257.
[56] Jin H M, Wang Q Q, Wu J W, et al. Baicalein inhibits the IL-1β- induced inflammatory response in nucleus pulposus cells and attenuates disc degeneration in vivo[J]. Inflammation, 2019, 42(3): 1032-1044.
[57] Jia J L, Nie L, Liu Y. Butyrate alleviates inflammatory response and NF-κB activation in human degenerated intervertebral disc tissues[J]. International Immunopharmacology, 2020, 78: 106004.
[58] Omote K, Hazama K, Kawamata T, et al. Peripheral nitric oxide in carrageenan-induced inflammation[J]. Brain Research, 2001, 912(2): 171-175.
[59] Tsuchida K, Ibuki T, Matsumura K. Bromoenol lactone, an inhibitor of calcium-independent phospholipase A2, suppresses carrageenan-induced prostaglandin production and hyperalgesia in rat hind paw[J]. Mediators of Inflammation, 2015, 2015: 605727.
[60] Moriyama T, Higashi T, Togashi K, et al. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins[J]. Molecular Pain, 2005, 1: 1744-8069.
[61] Studer R K, Vo N, Sowa G, et al. Human nucleus pulposus cells react to IL-6: independent actions and amplification of response to IL-1 and TNF-α[J]. Spine, 2011, 36(8): 593-599.
[62] George A, Schmidt C, Weishaupt A, et al. Serial determination of tumor necrosis factor-alpha content in rat sciatic nerve after chronic constriction injury[J]. Experimental Neurology, 1999, 160(1): 124-132.
[63] Schäfers M, Geis C, Svensson C I, et al. Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve[J]. The European Journal of Neuroscience, 2003, 17(4): 791-804.
[64] Bennett G J. A neuroimmune interaction in painful peripheral neuropathy[J]. The Clinical Journal of Pain, 2000, 16(3Suppl): S139-S143.
[65] George A, Buehl A, Sommer C. Tumor necrosis factor receptor 1 and 2 proteins are differentially regulated during Wallerian degeneration of mouse sciatic nerve[J]. Experimental Neurology, 2005, 192(1): 163-166.
[66] Schäfers M, Svensson C I, Sommer C, et al. Tumor necrosis factor-α induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons[J]. The Journal of Neuroscience, 2003, 23(7): 2517-2521.
[67] Shu X Q, Mendell L M. Neurotrophins and hyperalgesia[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(14): 7693-7696.
[68] Zhang J H, Huang Y G. The immune system: a new look at pain[J]. Chinese Medical Journal, 2006, 119(11): 930-938.
[69] Kajihara Y, Murakami M, Imagawa T, et al. Histamine potentiates acid-induced responses mediating transient receptor potential V1 in mouse primary sensory neurons[J]. Neuroscience, 2010, 166(1): 292-304.
[70] Ramírez-García P D, Retamal J S, Shenoy P, et al. A pH-responsive nanoparticle targets the neurokinin 1 receptor in endosomes to prevent chronic pain[J]. Nature Nanotechnology, 2019, 14(12): 1150-1159.
[71] Jensen D D, Lieu T, Halls M L, et al. Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief[J]. Science Translational Medicine, 2017, 9(392): eaal3447.
[72] Negri S, Faris P, Tullii G, et al. Conjugated polymers mediate intracellular Ca2+ signals in circulating endothelial colony forming cells through the reactive oxygen species-dependent activation of Transient Receptor Potential Vanilloid 1 (TRPV1)[J]. Cell Calcium, 2022, 101: 102502.
[73] Ang J M, Riaz I B, Kamal M U, et al. Photodynamic therapy and pain: a systematic review[J]. Photodiagnosis and Photodynamic Therapy, 2017, 19: 308-344.
[74] Hambly R A, Mansoor N, Quinlan C, et al. Factors predicting pain and effect of oral analgesia in topical photodynamic therapy[J]. Photodermatology, Photoimmunology & Photomedicine, 2017, 33(3): 176-179.
[75] Miller I M, Nielsen J S, Lophaven S, et al. Factors related to pain during routine photodynamic therapy: a descriptive study of 301 patients[J]. Journal of the European Academy of Dermatology and Venereology: JEADV, 2011, 25(11): 1275-1281.
[76] 周俊, 刘晶, 胡蝶, 等. 氟比洛芬酯在鲜红斑痣血卟啉单甲醚光动力疗法中镇痛效果的临床初步观察[J]. 中国皮肤性病学杂志, 2020, 34(5): 534-537.
Zhou J, Liu J, Hu D, et al. Analgesic effect of flurbiprofen ester in hematoporphyrin monomethyl ether-mediated photodynamic therapy for port-wine stains: preliminary clinical observations[J]. The Chinese Journal of Dermatovenereology, 2020, 34(5): 534-537.
[77] Borelli C, Herzinger T, Merk K, et al. Effect of subcutaneous infiltration anesthesia on pain in photodynamic therapy: a controlled open pilot trial[J]. Dermatologic Surgery, 2007, 33(3): 314-318.
[78] Chaves Y N, Torezan L A, Niwa A B M, et al. Pain in photodynamic therapy: mechanism of action and management strategies[J]. Anais Brasileiros De Dermatologia, 2012, 87(4): 521-529.
[79] Klein A, Karrer S, Horner C, et al. Comparing cold-air analgesia, systemically administered analgesia and scalp nerve blocks for pain management during photodynamic therapy for actinic keratosis of the scalp presenting as field cancerization: a randomized controlled trial[J]. British Journal of Dermatology, 2015, 173(1): 192-200.
[80] Skiveren J, Haedersdal M, Philipsen P A, et al. Morphine gel 0.3% does not relieve pain during topical photodynamic therapy: a randomized, double-blind, placebo-controlled study[J]. Acta Dermato-Venereologica, 2006, 86(5): 409-411.
[81] Liu L, Zhang Z H, Jiang X. Assessment of the analgesic effect of compound lidocaine cream in patients with port-wine stain treated by hemoporfin photodynamic therapy[J]. Proceedings of SPIE, 2019, 11070: 110707G.
[82] Holmes M V, Dawe R S, Ferguson J, et al. A randomized, double-blind, placebo-controlled study of the efficacy of tetracaine gel (AmetopR) for pain relief during topical photodynamic therapy[J]. British Journal of Dermatology, 2004, 150(2): 337-340.
[83] Anseline W, Grose D, Smith P, et al. A plant-derived anti-nociceptive spray for reduction of pain with photodynamic therapy[J]. Photodiagnosis and Photodynamic Therapy, 2014, 11(4): 467-471.
[84] Cabete J, Campos S, Lestre S. Conscious sedation with inhaled 50% nitrous oxide/oxygen premix in photodynamic therapy sessions for vulvar lichen sclerosus treatment[J]. Anais Brasileiros De Dermatologia, 2015, 90(1): 120-122.
[85] Fink C, Uhlmann L, Enk A, et al. Pain management in photodynamic therapy using a nitrous oxide/oxygen mixture: a prospective, within-patient, controlled clinical trial[J]. Journal of the European Academy of Dermatology and Venereology: JEADV, 2017, 31(1): 70-74.
[86] Raulin C, Greve B, Hammes S. Cold air in laser therapy: first experiences with a new cooling system[J]. Lasers in Surgery and Medicine, 2000, 27(5): 404-410.
[87] Pagliaro J, Elliott T, Bulsara M, et al. Cold air analgesia in photodynamic therapy of basal cell carcinomas and Bowen’s disease: an effective addition to treatment: a pilot study[J]. Dermatologic Surgery, 2004, 30(1): 63-66.
[88] Wennberg A M. Pain, pain relief and other practical issues in photodynamic therapy[J]. The Australasian Journal of Dermatology, 2005, 46(Suppl 3): S3-S4.
[89] Osiecka B J, Nockowski P, Szepietowski J C. Treatment of actinic keratosis with photodynamic therapy using red or green light: a comparative study[J]. Acta Dermato-Venereologica, 2018, 98(7): 689-693.
[90] Zeitouni N C, Paquette A D, Housel J P, et al. A retrospective review of pain control by a two-step irradiance schedule during topical ALA-photodynamic therapy of non-melanoma skin cancer[J]. Lasers in Surgery and Medicine, 2013, 45(2): 89-94.
[91] Wiegell S R, Haedersdal M, Philipsen P A, et al. Continuous activation of PpⅨ by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single-blinded study[J]. The British Journal of Dermatology, 2008, 158(4): 740-746.
[92] 林立, 李步洪. 发光二极管在光动力疗法中的应用进展[J]. 激光与光电子学进展, 2020, 57(15): 150001.
[93] Gholam P, Bosselmann I, Enk A H, et al. Low irradiance compared with conventional photodynamic therapy in the treatment of actinic keratoses[J]. Photodermatology, Photoimmunology & Photomedicine, 2019, 35(2): 110-115.
[94] Cottrell W J, Paquette A D, Keymel K R, et al. Irradiance-dependent photobleaching and pain in delta-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas[J]. Clinical Cancer Research, 2008, 14(14): 4475-4483.
[95] Wulf H C. The background and philosophy behind daylight photodynamic therapy[J]. Giornale Italiano Di Dermatologia e Venereologia, 2018, 153(6): 776-782.
[96] Nguyen M, Sandhu S S, Sivamani R K. Clinical utility of daylight photodynamic therapy in the treatment of actinic keratosis-a review of the literature[J]. Clinical, Cosmetic and Investigational Dermatology, 2019, 12: 427-435.
[97] Wiegell S R, Wulf H C, Szeimies R M, et al. Daylight photodynamic therapy for actinic keratosis: an international consensus[J]. Journal of the European Academy of Dermatology and Venereology: JEADV, 2012, 26(6): 673-679.
[98] Fink C, Enk A, Gholam P. Photodynamic therapy-aspects of pain management[J]. JDDG: Journal Der Deutschen Dermatologischen Gesellschaft, 2015, 13(1): 15-22.
Article Outline
雷栋钦, 刘晶, 张镇西, 曾维惠, 姚翠萍. 光动力疗法产生疼痛的机制以及临床干预手段[J]. 中国激光, 2023, 50(9): 0907206. Dongqin Lei, Jing Liu, Zhenxi Zhang, Weihui Zeng, Cuiping Yao. Mechanism of Pain in Photodynamic Therapy and Clinical Interventions[J]. Chinese Journal of Lasers, 2023, 50(9): 0907206.