Castable solid pressure media for multianvil devices
1 INTRODUCTION
Generating high pressures for phase equilibrium studies, material synthesis, and the investigation of material properties under extreme conditions has led to significant advances in the study of Earth and planetary interiors, solid-state physics and chemistry, and materials sciences. The conversion of motive force to pressure requires a pressure transmitting medium. A simple case is the use of a pump to increase the fluid content within a filled, closed vessel. The fluid is the pressure medium, and the pressures produced are hydrostatic. Gaseous fluids store the mechanical work of compression, so that it becomes increasingly challenging at high compressions to deliver sufficient gas. Gas leaks can release that stored energy unsafely. Liquid pressure media of lower compressibility than gases are thus advantageous at high pressures. However, fluids may encounter freezing at high pressures, in which case the solid pressure media no longer transmit pressure to the vessel contents in a hydrostatic manner. If frozen pressure transmitting media are not too strong in shear, the deviation from hydrostatic pressure may be acceptable. The use of weak deformable solids as pressure transmitting media in very high-pressure devices can be more convenient than fluids. Machinable ceramics like Macor, talc, pyrophyllite, and hexagonal boron nitride have been used as pressure media. Die-formed granular aggregates of NaCl, KCl, BaCO3, and CaF2 are also used in cylindrical geometry applications.
The multianvil apparatus has been widely used to generate pressures between tens of kilobars (a few gigapascals) up to 1 Mbar, as reviewed by Liebermann.
2 CERAMACAST 646
The difficulties with the Ceramacast 584 pressure medium motivate and inform a search for alternative castable materials for use as pressure media. This report reviews some characteristics of the zirconia-based castable Aremco compound 646. We determine pressurization efficiency, both hot and cold, as well as the thermal structure within LaCrO3 (LCO) heaters that are placed inside 646 octahedra. The pressurization efficiency of 646 is still not competitive with 584, confirming the results from 1991. However, the ∼20%–30% lower pressurization efficiencies are compensated by convenience in preparation and fabrication of 646 assemblies compared to the “fussy” 584, including longer pot life (tens of minutes), longer shelf life (years), simpler curing and firing, and excellent structural integrity when machined. Moreover, the zirconia 646 pressure medium is far superior to 584 in its thermal insulation characteristics. Good thermal insulation is desirable for keeping the tungsten carbide anvils cool and for promoting a uniform thermal structure of the payload, but is undesirable for achieving rapid quenching rates. If lower pressurization efficiency and quench rate are acceptable, Aremco Ceramacast 646 provides an attractive alternative to 584 for use as a multianvil device pressure medium.
Although octahedra with precast fins were initially introduced to eliminate the need for making and installing separate pyrophyllite gaskets, the 646 material can be cast as “normal” finless octahedra and be used in combination with pyrophyllite gaskets. Finless 646 offers an alternative to the finless octahedra from Japan Engineering Co. and the now widely used injection molded octahedra developed by COMPRES,
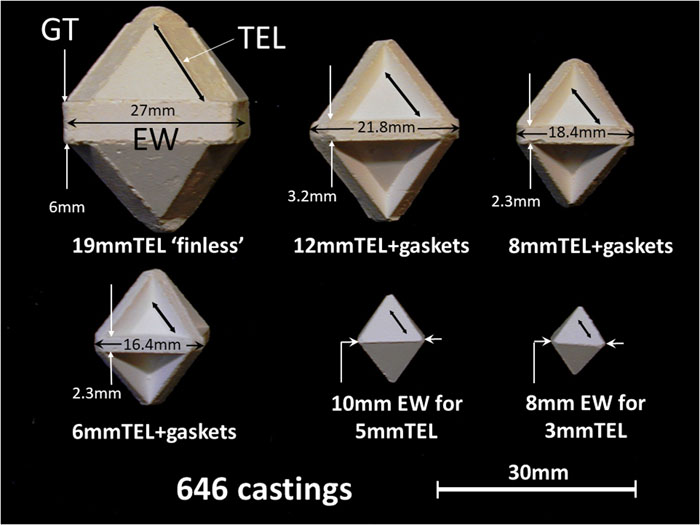
Fig. 1. Ceramacast 646 pressure media for use in multianvil pressurizations. Dimensional abbreviations: TEL = truncated edge length of the triangular corner facet on a tungsten carbide anvil, which will mate to the pressure medium’s triangular octahedral face. GT = gasket thickness. EW = equatorial width. Regular octahedra of 10 mm and 8 mm EW without integral cast gaskets are for mating to 5 mm and 3 mm TEL anvils with COMPRES pyrophyllite gaskets.
A note on the preparation of Ceramacast 646 for application as a pressure medium in heated experiments: 646 must be rigorously dewatered by heating, just as Ceramacast 584 must be. Ignore the curing recipe on the label supplied by Aremco. Instead, heat the unfired casting at about 1000 °C/hour from room temperature to 1050 °C and hold at 1050 °C for at least an hour. Do not put the casting directly into a 1050 °C furnace as crumbling may occur. Firing for 2 h instead of 1 h does no damage. After a fresh pour of 646 has cured at least overnight in the molds, it can be fired immediately or stored, without any special humidification, for indefinite periods of time before firing. The firing step, whether performed or not, makes no difference to the results of Bi calibrations, but is required for applications at high temperature where water escape during heating can catastrophically destabilize the experiment. Be warned.
3 CALIBRATIONS
The calibration results are given in the form of sample pressure in kilobar (and gigapascal) vs press load in US tons produced by the application of oil pressure to a hydraulic ram. Other units of pressure may be used in various laboratories. The bar is a metric unit of pressure, defined as exactly equal to 100 000 Pa, which is slightly less than the current average atmospheric pressure on Earth at sea level. The metric ton, also known as “tonne,” equals 1000 kg, or approximately 2204 pounds. The US ton, also known as the short ton, is 2000 pounds, whereas the British ton is the long ton, which is 2240 pounds.
4 IN SITU BI CALIBRATION AT ROOM TEMPERATURE
Table 1. Room temperature Bi calibration in cast octahedra with integral fin gaskets.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
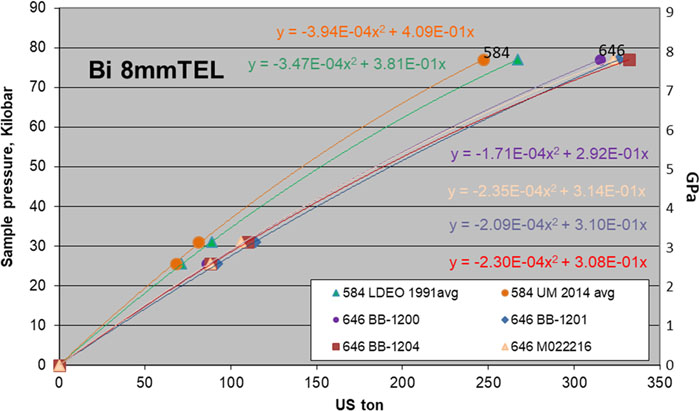
Fig. 2. 8 mm TEL Bi calibrations at room temperature. Ceramacast 646 (red, purple, blue, and pink) are compared with 584 (green and orange).
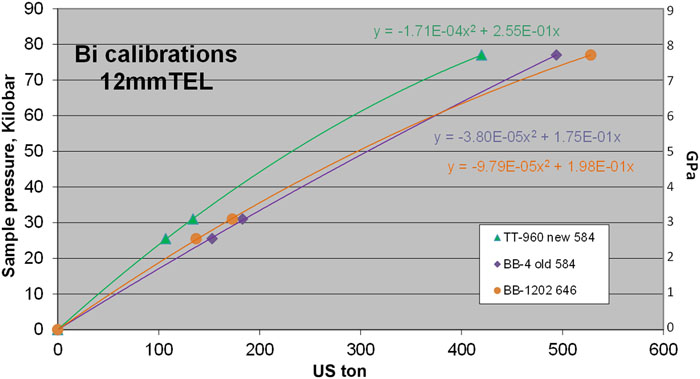
Fig. 3. 12 mm TEL Bi calibrations at room temperature. Ceramacast 646 (orange) is compared to 584 (green and purple).
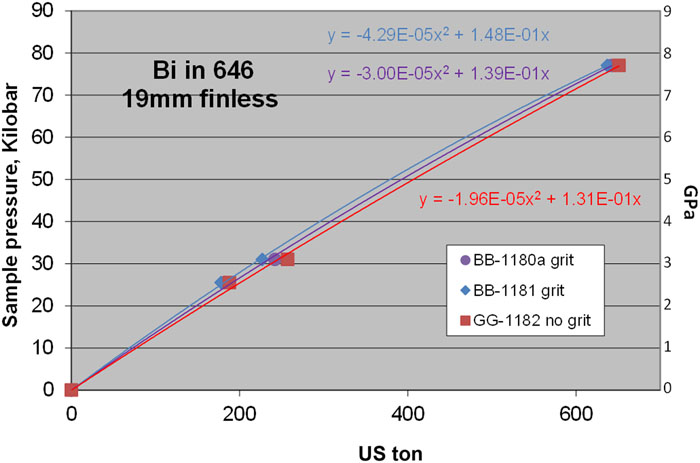
For the 19 mm finless assemblies shown in
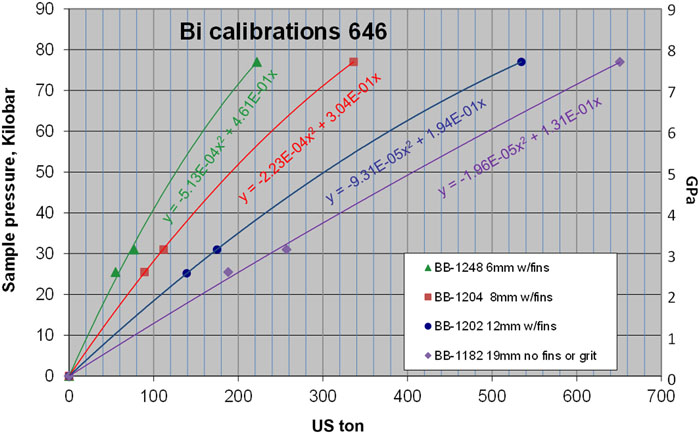
Putting all 646 Bi room temperature results together in
There is a regular spacing or proportionate gap between the 6 mm and the 8 mm results as pressure increases (
5 HIGH-TEMPERATURE CALIBRATIONS
Pressure calibration of multianvil presses typically uses multiple quenched experiments to bracket phase transitions at selected pressures at high temperature. More recently, Li and Li
Table 2. High temperature SiO2 and CaGeO3 calibrations in cast octahedra with integral fin gaskets.a
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 3. High temperature Mg2SiO4 calibrations in finless octahedra with pyrophyllite gaskets.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 4. High temperature NaCl melting calibrations in octahedra with integral fin gaskets for 8 mm TEL WC anvils.a
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
6 LOAD-AND-QUENCH CALIBRATION OF SOLID-STATE PHASE TRANSITIONS
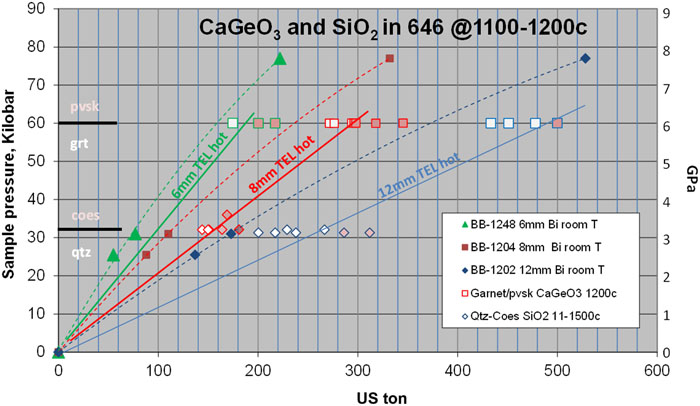
Fig. 6. High-temperature load-and-quench calibrations for CaGeO3 and SiO2 in 646. Abbreviations: quartz (qtz), coesite (coes), garnet (grt), perovskite (pvsk).
The identification of garnet or perovskite polymorphs of CaGeO3 in the experimental products was initially determined from vague morphological suggestions of cubic or dodecahedral habit in a polished section. The results were confirmed by distinct Raman spectra of the polished grains recovered at room pressure and temperature (
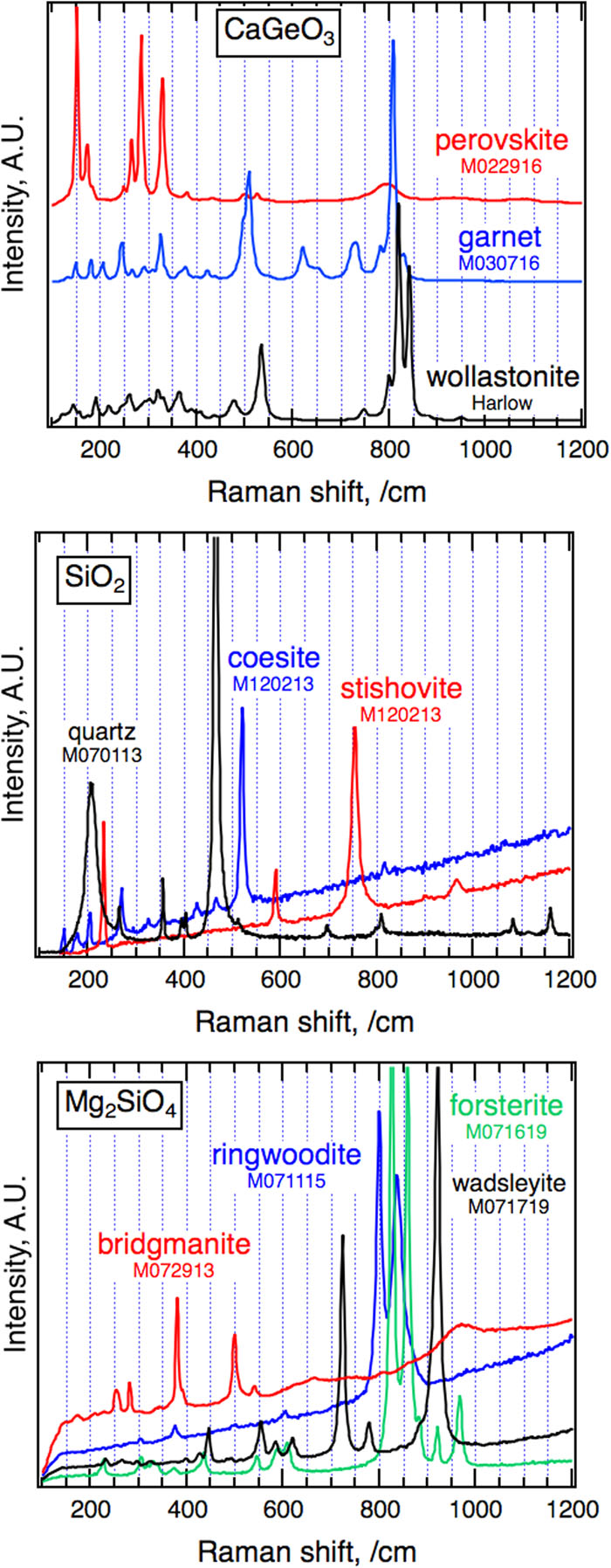
Fig. 7. Raman spectra of starting materials and products of calibration experiments. The spectra were collected using a Renishaw Raman microscope, with 532 nm unpolarized laser light, a 50× objective (2 μ m area) in the nonfocal mode, and an 1800 l/mm grating. The collection time ranged between 1 and 60 s. Results are shown without background subtraction.
The ambiguities of crystal morphology and the possible unavailability of IR or Raman spectroscopy provide an incentive for finding additional diagnostic criteria for polymorphs of CaGeO3. There is a simpler diagnostic test for the recovered polymorph which did not require any sample preparation. The garnets are bright white and the perovskites are pale tan/pink, for charges wrapped in Pt foil, a fact that should aid the determination of pressure calibrations with CaGeO3, as seen in
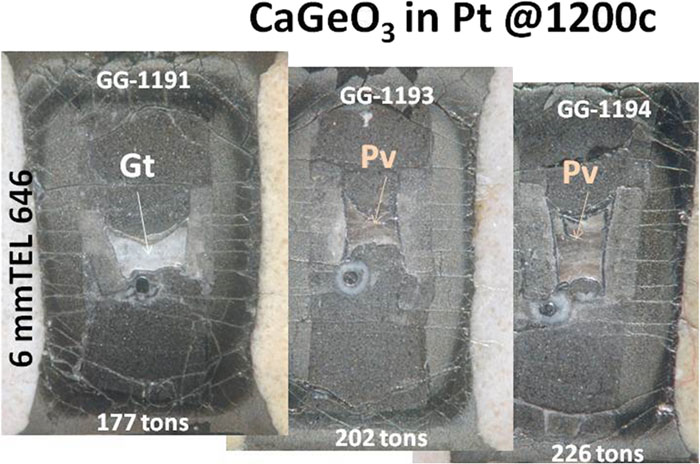
Fig. 8. CaGeO3 polymorphs wrapped in Pt display color differences: garnet is white, perovskite is tan/pink.
Pressure calibrations of 5 mm and 3 mm TEL using pyrophyllite gaskets on COMPRES or 646 octahedra are seen in
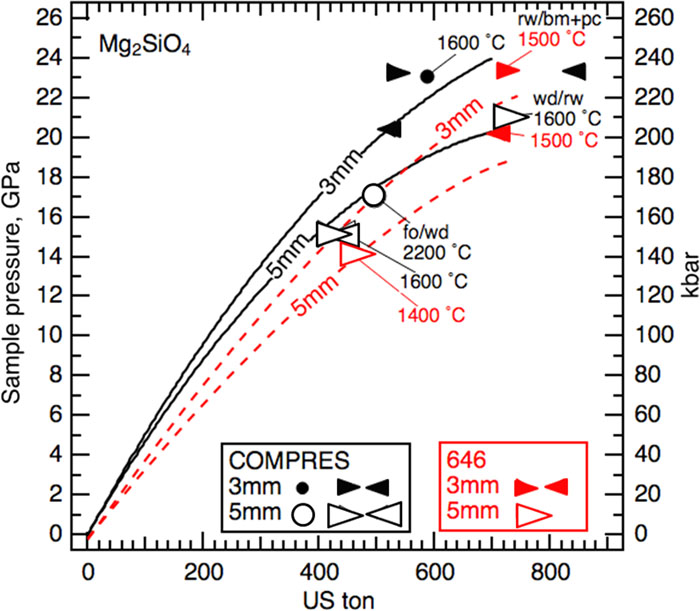
Fig. 9. High temperature calibrations using Mg2SiO4 starting materials for 3 mm and 5 mm TEL octahedra with pyrophyllite gaskets. Data are found in Table III . The results of experiments using COMPRES injection molded octahedra (black symbols) are in good agreement with the black curves reproduced from Leinenweber et al. 3 The results of experiments using 646 cast octahedra (red symbols) are used to constrain the red dashed curves. Circles denote two phases coexisting. Left- and right-pointing wedges denote single phases at higher tonnage or lower tonnage, respectively, than the relevant phase boundary at the experimental temperature. In experiment M080319, ringwoodite was the product at 715 tons and 1500 °C. The two red-filled wedges indicate that the tonnage was above that required for the rw/wd transition at 20.3 GPa and below that required for the rw/bm + pc transition at 23.3 GPa. Thus, sample pressure is greater than the 20.3 GPa needed for the rw/wd boundary and less than the 23.3 GPa needed to achieve the rw/bm + pc boundary. Abbreviations: forsterite (fo), wadsleyite (wd), ringwoodite (rw), bridgmanite (bm), periclase (pc).
7 IN SITU CONDUCTIVITY CALIBRATION ALONG A MELTING CURVE
On the basis of an established melting curve of NaCl,
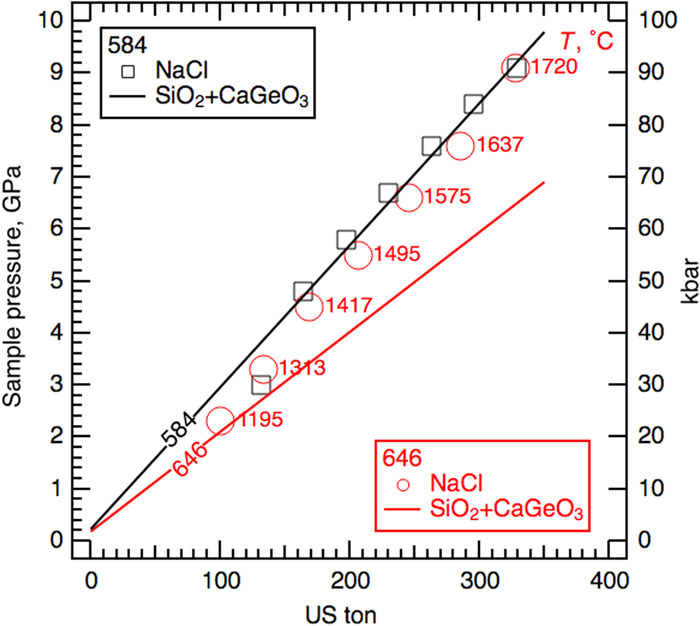
Fig. 10. Pressure calibrations of 8 mm TEL anvils based on the melting curve of NaCl. Black (584) and red (646) lines are linear fits to load-and-quench calibration experiments using SiO2 and CaGeO3 (Table III ). Red circles and black squares represent pressures determined from the melting temperatures of NaCl (marked next to the 646 data points) at various press loads.
All the experiments were conducted using the 1000-ton Walker-style multianvil press at the University of Michigan and used Re heater and Fansteel tungsten carbide (WC) cubes. As described by Li and Li,
In the 584 experiment (M100313,
The 646 experiment (M022219) used a type-S thermocouple of 0.20 mm diameter to monitor the temperature. The electrode and thermocouple wires were inserted into single four-bore alumina tubing, which was then inserted axially into the cylindrical heater from one side. The electrode tips and thermocouple junction were located in a plane parallel to and near the heater equator. This configuration ensured that the temperature and conductivity signals originated from the same region of the sample. Press loads were selected to produce 3–9 GPa sample pressure with 1 GPa intervals, for 584 pressure media, according to the results of load-and-quench calibration experiments using SiO2 and CaGeO3 transitions (
The pressure of NaCl at a given press load is calculated from the measured melting temperature using Simon’s equation (T/T0)c = (P − P0)/A +1, where T0 = 1073.6 K and T (in Kelvin) are the melting temperatures at the reference pressure P0 = 1 bar = 0.0001 GPa and P (in gigapascal), respectively. Fitting the reported data of Akella et al.
For 584, the NaCl pressures match well with the load-and-quench, single cycle calibration line, except at 140 ton, where the NaCl pressure is lower by more than 1 GPa (
A previous study using in situ synchrotron x-ray diffraction showed that, at a fixed press load, sample pressure decreased by 1–2 GPa when the sample was heated from 1200 °C to 1600 °C (Fei et al.
While the in situ conductivity calibration method using NaCl has the distinct advantage of high efficiency and more precise bracketing, its resolution diminishes at high pressures where the melting slope becomes small, and therefore, the melting temperature becomes less sensitive to pressure. With NaCl, the melting slope changes from 200 °C/GPa at low pressures to 20 °C at 15 GPa, and therefore it would no longer be a good candidate for pressure calibration above 10 GPa.
8 THERMAL STRUCTURE
The superior thermal insulating properties of 646 castable ceramic reflect its zirconia base. The thermal conductivity of ZrO2 (1.8–2.2 W/m-K) is ten times smaller than that of Al2O3 (40 W/m-K) or MgO (28–35 W/m-K) at 1 bar and 300 K (Clauser and Huenges
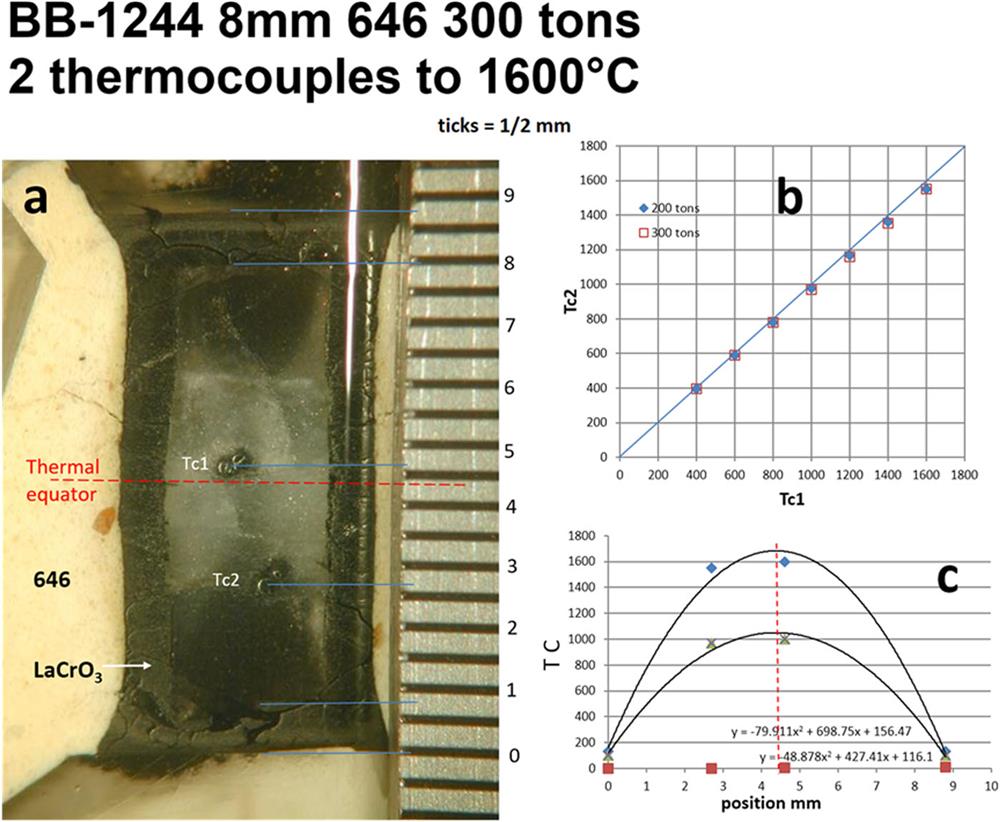
Fig. 11. Thermal structure in 8 mm 646 assembly with LaCrO3 heater determined by two independent thermocouples 2 mm apart.
9 SUMMARY
Castable ceramics, compared to machined or injection molded pressure media, can be fabricated into intricate shapes without undue repetitive effort once a mold has been formed. Their ability to be cast with integral gasket fins avoids the steps of cutting and placement of pyrophyllite gaskets. This simplifies the assembly of experiments and reduces the complexity of the supply chain. Different castable compounds have different strengths and weaknesses. Our exploration over the years of Ceramacast 584 and 646 reveals that, in general, 584 is superior in efficiently translating press force into sample pressurization; in some cases, it is slightly more efficient than the standard machined or injection molded multianvil pressure media with pyrophyllite gaskets. The appeal of the less pressure-efficient 646 includes its ease of casting and fabrication. Its superior thermal insulation conserves power and protects the anvils and lubricating plastic pads from thermal damage at high experiment temperatures better than the alternatives. However, the performance of castable 584 and 646 ceramics with integral fin gaskets above 10 GPa is not as reliable as the standard ceramic+pyrophyllite gasket assemblies in preventing blowouts. This suggests the use of castable ceramic octahedra with pyrophyllite gaskets as a substitute for the more costly machined or injection molded standard materials at pressures above 10 GPa. Our study shows that even the pressure-inefficient 646, when used with pyrophyllite gaskets, can be competitive with other standard materials in pressure generation at lower cost and with better thermal insulation.
[2] . Lubrication, gasketing and precision in multianvil experiments. Am. Miner., 1991, 76: 1092.
[4] . High-pressure calibration: A critical review. J. Phys. Chem. Ref. Data, 1972, 1: 773.
[12] . Melting of sodium chloride at pressures to 65 kbar. Phys. Rev., 1969, 185: 1135.
[14] . Phase diagram of Bismuth to 130 000 kg/cm2, 500 °C. Phys. Rev., 1958, 110: 314.
[15] . The pressures of some solid-solid transitions. J. Geophys. Res., 1962, 67: 851.
Article Outline
David Walker, Jie Li. Castable solid pressure media for multianvil devices[J]. Matter and Radiation at Extremes, 2020, 5(1): 018402.