1 I. INTRODUCTION
In 1971, the Nobel Prize winning academician Vitaly Ginzburg compiled a list of the most important and interesting questions in physics and astrophysics facing us on “the verge of XXI century.”1 The first and second problems in the list were controlled nuclear fusion and room temperature superconductivity, and the third was metallic hydrogen. How the simplest element in the Universe could transform into a dense metal has turned out to be one of the most interesting and fundamental questions in condensed matter science. In the past 20 years, many of the problems Ginzburg outlined have already been solved, leading to Nobel Prizes: Bose–Einstein condensates,2 the discovery of the Higgs boson,3 and the discovery of gravitational waves4 and the development of new types of astrophysical observations based on them. However, all evidence to date suggests we have still to reach the solid metallic state of hydrogen. The fact that the seemingly simple problem of hydrogen metallization has not yet been solved reflects the experimental difficulties associated with dealing with the material at high densities.
But why did Ginzburg place the problem of metallic hydrogen on a par with Bose–Einstein condensates or room temperature superconductors? Hydrogen is the most common atom in the visible Universe. With one electron, it exists in a molecular state at ambient conditions and readily forms compounds with almost every other element in the periodic table. If combined with oxygen, it forms water, the main requirement for life to exist; if combined with lanthanum, it forms LaH10, which to date has the highest claimed temperature of superconductivity (Tc = 260 K at a pressure of 180 GPa).5 It is thought that highly condensed metallic hydrogen is the main constituent of the Jovian planets, such as Jupiter, and is responsible for the dynamo driving their extraordinary planetary magnetic fields.6 Here on Earth, fusion of hydrogen isotopes is widely seen as the only energy source capable of powering advanced societies over millennium timescales. Even today, hydrogen fuel cells are already being used in public transport systems. Being the first element of the Periodic Table and deceptively the simplest element, hydrogen represents a classical testing ground for many fields of science: physics, chemistry, geosciences, and material sciences. The current known phase diagram of hydrogen (see Fig. 1), combined with the predicted unusual properties such as superconductivity or superfluidity that might exist at very high compressions, make it an obvious subject to study in solid state physics and chemistry. Knowledge of its solid phases and their structures and of its optical properties helps theory in creating effective potentials and in testing current theoretical models, while knowledge of its interaction with other elements can guide chemical physics in the search for novel compounds with interesting properties.
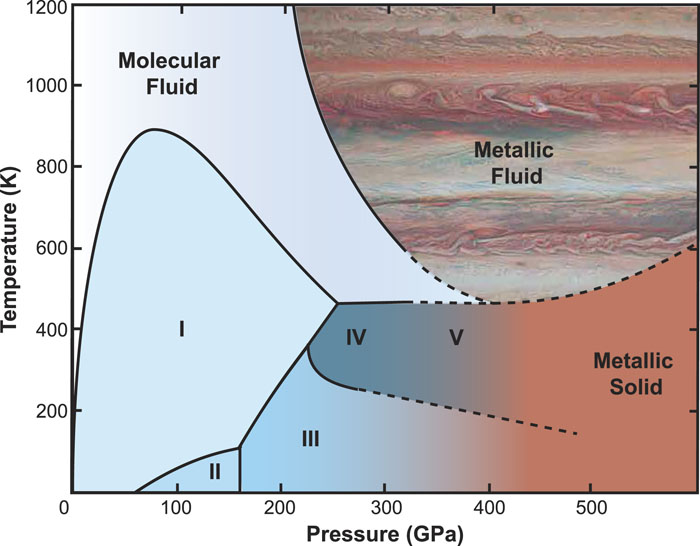
Fig. 1. Proposed (artistic) P–T phase diagram of H2. Solid phase lines are a combination of static compression studies of solid hydrogen9–13 and dynamic compression studies of fluid deuterium.14,15 Dashed lines represent extrapolations of these combined results. The dark brown color of phases III and V at higher pressures suggests closing of the bandgap.
下载图片 查看所有图片
The “metallic hydrogen problem” was actually formulated much earlier than the paper by Ginzburg in 1971 (of which Ref. 1 and the Nobel Lecture7 are updated versions). In 1935, Eugene Wigner (one of the founders of modern solid-state physics) and his colleague Hillard Huntington first tried to predict what would happened to hydrogen if it were compressed to very high densities.8 Based on a nearly free-electron picture, they predicted that above 250 000 atm (25 GPa)—an unimaginable pressure at the time—hydrogen would enter a metallic state. Because they did not know the compressibility of hydrogen, they were quite far off in their estimate of the pressure required. Experimental high-pressure physics has developed and matured over the eight decades since, succeeding in subjecting hydrogen to pressures of the order of 400 GPa,9 an almost 16-fold increase compared with the original prediction of Wigner and Huntington. A plethora of exciting and interesting phenomena have been observed in dense hydrogen, but the metallic state remains elusive. Owing to the accumulated experience, knowledge, and significantly improved experimental and theoretical methods, we now understand the problems much better and can make an educated guess as to the P–T conditions needed to turn the molecular gas into the lightest metal. While the experimentalists are tantalizingly close to the pressures needed to metallize hydrogen, theory has already moved beyond current static pressure limits and has predicted that ground-state (T = 0 K) hydrogen, owing to strong quantum effects, would be an entirely new state of matter, which could be superfluid or superconducting, depending on the magnetic field applied.16 This fascinating prospect is so unusual that it is quite difficult to imagine it being possible. Consequently, metallizing hydrogen and reaching such a novel state of matter is arguably the most exciting and interesting discovery that condensed matter physics could produce today.
2 II. PHYSICS OF DENSE HYDROGEN AND DEUTERIUM AT HIGH DENSITIES (COMPRESSION)
The behavior of hydrogen is strongly influenced by quantum mechanical effects. Nuclear quantum effects are larger for hydrogen than any other atom, which explains its unique behavior. Solid hydrogen has a massive quantum zero-point energy (ZPE), far greater than its latent heat of melting, and has a Debye temperature well above melting. These factors determine the behavior of hydrogen in the dense state. Currently, five solid phases of hydrogen are known (see Fig. 1), and it is unique among the stable elements in that full structural information (e.g., the locations of the atomic centers and the shapes of the molecules) is absent for all of them, which prevents modeling and/or predictions of hydrogen behavior at higher pressures.
Under ambient conditions, i.e., atmospheric pressure and 300 K, hydrogen is a molecular gas [see Fig. 2(a)]. The exchange interaction, a purely quantum mechanical effect, forms one of the strongest bonds in chemistry, the H–H bond. Owing to this bond, hydrogen exists in molecular form, with atoms separated by approximately 0.74 Å and a bond dissociation energy of approximately 4.52 eV under ambient conditions.17,18 In its solid state at 2 K, the hydrogen bandgap is very large, at about 14 eV.19 Conversely, intermolecular bonding is very weak, requiring extreme conditions to bring the molecules together and bind them into the solid state. Low-temperature solidification of hydrogen was first achieved in 1899 by Dewar, at a slightly higher temperature (19 K) than that required to liquefy helium. An alternative solidification route is through compression, whereby hydrogen can be solidified at 300 K by bringing the molecules close to each other and increasing the density. The gaseous, diffusive, and corrosive nature of hydrogen, combined with the lack of high-pressure technology, delayed room temperature solidification for almost a century after Dewar’s experiments. Only the invention and refinement of the diamond anvil cell allowed Mao and Bell20 to solidify hydrogen at 300 K using a pressure of 5.5 GPa (55 000 atm). The solid state under these conditions is now known as phase I (Fig. 1). This phase is characterized by quantum spherically disordered molecules arranged in a hexagonal close packed (hcp) structure [Fig. 2(b)]. At room temperature and above 5.5 GPa, hydrogen is a very good (molecular) insulator with a bandgap of 9.5 eV (H.-K. Mao, unpublished work). Phase I occupies a very prominent part of the phase diagram, reaching up to 190 GPa at 300 K. It displays remarkable pressure stability and to our knowledge extends over the second largest pressure range for any molecular system, being second only to molecular chlorine, whose phase I exists over a pressure interval of 230 GPa.21 Phase II, known as the “broken symmetry” phase,23 is formed by compressing phase I of hydrogen or deuterium above 60 GPa or 25 GPa, respectively,13 and at temperatures below ∼100 K. Governed by quantum effects, phase II is thought to have ordered (or at least partially ordered) molecules, but the nature of their arrangement and their shape are unknown.24 There is a strong isotope dependence in the transition from phase I to II, with the deuterium transition occurring at substantially lower pressures than that in hydrogen, implying a critical role of nuclear quantum effects. Phase III is obtained by compressing phase II above ∼155 GPa below 100 K25 or at around 190 GPa at 300 K10,11 (see Fig. 1). Nothing so far is known about its structure (atomic positions), but it has been shown to also have an hcp lattice,26,27 with unusually intense infrared activity.28 It has very recently been shown that phase III extends over a pressure interval of more than 200 GPa at low temperatures.22 The phase diagrams of hydrogen and deuterium were studied in great detail in the 1990s, leading to many interesting discoveries: for example, both isotopes have a triple point, i.e., a P–T point at which the three phases, I, II, and III, meet.29 However, for the next two decades, the highest pressures to which hydrogen was subjected were limited to about 300 GPa at low temperatures30,31 and only 160 GPa at room temperature, owing to the diffusive and reactive nature of the material in a dense state.32
Fig. 2. Artistic representation of the gaseous and solid states of hydrogen under different pressures at room temperature (300 K): (a) gaseous molecular state; (b) phase I, with hcp structure; (c) phase IV, with mixed molecular and atomic state; (d) purely atomic and metallic state.
下载图片 查看所有图片
It took almost 25 years from the discovery of phase III to the observation of phases IV of hydrogen and deuterium.10,33 If phase III is compressed at 300 K, it transforms into phase IV at around 230 GPa. Phase IV is thought to be entropy-driven and is arguably (together with phase V, described below) the most unusual phase of hydrogen. Even though the structure of phase IV is not known, on the basis of Raman spectroscopy combined with theoretical structural searches, it has been speculated that it is made up of alternating layers consisting of six-atom rings and free-like molecules.10,34 The interatomic distances in the ring are around 0.82 Å, leading to a vibrational frequency of around 2700 cm−1, which is significantly reduced compared with that under ambient conditions, while the atoms in the free-like molecules have a vibrational frequency close to 4200 cm−1. A recent x-ray diffraction study27 has demonstrated the persistence of hcp symmetry into phase IV, despite the observed fundamental changes in optical properties.
If phase IV is further compressed at 300 K, it gradually transforms into phase V,9 with the transformation lasting over a range of 50–60 GPa, starting at 275 GPa and effectively finishing at above 325 GPa. Interestingly, owing to the differences in quantum mechanical properties between hydrogen and deuterium, phase V has not been observed in the latter. Phase V has been speculated to be a partially purely atomic state and a precursor to a fully metallic and atomic state.9
3 III. DISSOCIATION AND METALLIZATION
Building on the earlier prediction by Wigner and Huntington,8 Abrikosov,35 and others,36,37 Ashcroft theorized in his seminal paper38 that if the hydrogen molecule is dissociated and a purely atomic alkali-metal-like solid is formed, this solid could exhibit room temperature superconductivity. In fact, the first experiments to break the hydrogen bond were attempted by Langmuir39 more than 100 years ago. They demonstrated that extreme conditions are indeed needed to do so; for example, the H2 molecule dissociates only to a minor extent at high temperatures (at 3000 K, the degree of dissociation is around 10%).40 Another mechanism to break the hydrogen bond is to employ another thermodynamic variable, namely, pressure, exactly as Wigner and Huntington suggested some years after the Langmuir experiments. However, the proposed high-pressure route to an atomic metallic state has proved to be one of the great experimental challenges in high-pressure physics, and, despite technological advances, this theoretical prediction has yet to be experimentally confirmed. Hydrogen is expected to become metallic and also nonmolecular, but the pressure at which this occurs is not known precisely, nor is it known whether metallization and dissociation occur simultaneously. However, the recent discovery and study of phase V has provided the first experimental suggestion that dissociation will be accompanied by metallization and that both effects happen simultaneously and gradually as pressure is increased.9
The insulator-to-metal transition in liquid deuterium has recently been claimed to have been observed in shock-wave experiments.14,15 However, the observed metallic liquid state of deuterium exists at relatively high temperatures (roughly around and above 1000 K15), which is not the ground liquid state of the system predicted theoretically. In this paper, we focus only on metallic states of hydrogen (and deuterium) and their properties at “low” temperatures, namely, around and below 300 K.
Shortly after hydrogen was solidified in the diamond anvil cell, it was studied by Raman spectroscopy to around 66 GPa.41 This study found that the intramolecular vibrational frequency of hydrogen increases with pressure up to 33 GPa, but then starts to decrease as more pressure is applied. Since vibrational frequency is a measure of H–H bonding strength, one can easily extrapolate that at some very high pressure, the bond will be broken and molecular hydrogen can transform into an alkali-like free-electron metal similar to Li or Na. As pointed out by Sharma et al.,41 “the increase in frequency becomes less and finally decreases at approximately 330 kbar, as the molecular bonds are weakening. Eventually when molecular hydrogen transforms to the predicted atomic (metallic) state, the molecular bonds will be broken.”
Although the sample environment of the diamond anvil cell is restricting, there are several probes that can be used to evaluate the degree of “metallicity.” However, all of these probes have their limitations, which, taken together with the small linear size (2–3 µm) of the hydrogen samples required to reach pressures above 350 GPa, can easily lead to misinterpretation of the data, and in turn to erroneous claims of metallization.
The very first claim of hydrogen metallization was made in 1989 by a group from the Geophysical Laboratory at the Carnegie Institute, who, on the basis of the diminishing Raman signal and increased absorption by the sample, concluded that they had reached the metallic state somewhere above 200 GPa.42 This was soon followed by another claim from a group from Harvard University.43 However, with improvements in experimental methods, it became apparent that the observed effects (e.g., loss of Raman signal and “darkening” of the sample) could be explained by loss of hydrogen at high pressures and by increased fluorescence of the diamonds being mistaken for closing of the bandgap.
About 20 years later, there was another claim of metallization, when, combining Raman spectroscopy with direct electrical measurements of sample resistance, a group from the Max-Planck Institute made the bold claim that they had observed “liquid atomic metallic hydrogen” at above 260 GPa.33 The claim was yet again based on the disappearance of the Raman signal and on an abrupt drop in sample resistivity at 260 GPa. However, almost immediately after publication of this paper, it was shown that hydrogen remains in a mixed molecular and atomic semiconducting solid (phase IV) phase to at least 315 GPa at 300 K,10 transforming to phase III at lower temperatures.11 The loss of Raman signal and the drop in sample resistance were explained by loss of hydrogen and collapse of the sample chamber.44
After the discovery of phase V above 325 GPa,9 and the suggestion that this phase could represent the onset of dissociation and the first step toward a completely metallic state,9,45 the claims of metallization and extremely high pressures reached in experiments started to pick up pace (with three claims in the past three years). Among the many metallization claims over the past three decades, a recent paper by the Harvard group was arguably the most widely discussed owing to its somewhat outlandish statements, such as the suggestion that metallic hydrogen produced on a picoliter scale at 500 GPa would be a good candidate for a rocket fuel.46 Even the title of the paper, “Observation of the Wigner–Huntington transition to metallic hydrogen,” is misleading, because the Wigner–Huntington transition is a transition between molecular and atomic states, whereas the paper did not demonstrate either molecular or atomic states of hydrogen. Since the claims of metallization and an extremely high pressure of 500 GPa (which is currently widely believed to be just outside of the range of the standard diamond anvil cell techniques) were not accompanied by any scientific evidence other than iPhone photographs, four comments criticizing the work immediately followed47–50 and even generated a public debate on metallic hydrogen.51
Currently, there is no general agreement in the high-pressure hydrogen research community on the behavior of hydrogen (and its isotopes) above 250 GPa: for example, there is disagreement even on the phase diagram and the phase labeling.9,22,46,52 There is also a clear disagreement as to whether the metallic state was reached and at what pressures. The ultimate study will have to include robust evidence of metallization based on techniques that directly probe the electronic state of the sample, i.e., electrical measurements or measurements of reflectivity/transmission. Even if these techniques are used, one needs to make sure that the data are reliable and reproducible. For example, during electrical measurements, the electrodes will form a metallic hydride on contact with hydrogen, which could mask the real value of the resistance, or the defusing hydrogen could cause the sample chamber to collapse or change shape, thereby moving and/or shortening the electrodes, as indeed happened in one of the earlier measurements.33 Reflectivity/transmission measurements are also nontrivial owing to the extremely small size (2–3 µm in linear dimensions) to which the sample collapses at around 400 GPa, changing the geometry of the sample chamber and thus precluding proper reference measurements. The claim of metallization at 500 GPa from the Harvard group46 was based on two wavelength points measured after the metallic state had supposedly been reached (four different wavelengths were measured, but two of them were later retracted53). With the lack of measurements at lower pressure, of raw reflectivity data, and of any transmission data at all, the claim of metallization must be placed under question.
To allow comparison of the results from different groups, one needs to have reliable pressure measurements. Currently, it is accepted that pressures above 400 GPa are close to the limit of the standard diamond cell configuration.47–50 The pressures are usually estimated from the shift in the Raman mode of diamond, which in turn can be cross-referenced with the signal from the sample.44 The Raman frequency of the hydrogen vibrational mode must be used to give more reliable connections among different experiments than are obtained using the diamond mode shift: with the hydrogen mode shift, the state of the sample is probed directly, which is not the case with the diamond mode shift.44 For infrared reflectivity/transmission measurements, the counterpart infrared vibrational frequency could be measured and cross-referenced with the diamond shift, which would allow comparison of pressures among different experiments. For instance, within the 500 GPa pressure range, Ref. 46 provided only four pressure measurements points using three different non-overlapping techniques, extrapolating the pressure from ∼300 GPa to 500 GPa. Not a single measurement directly related to the sample was presented. Another example of unconvincing pressure measurements is the latest paper claiming semimetallic hydrogen up to 480 GPa.22 The presented diamond shift is indistinguishable from the background above 420 GPa, while the hydrogen vibrational mode disappeared at 372 GPa, posing legitimate questions as to whether the provided pressures are correct.
The most important factor when dealing with such a hot topic as hydrogen metallization is reproducibility. Almost all of the debunked claims of hydrogen metallization have each been based on a single unconvincing experiment that was never later confirmed. The huge experimental research effort that is required to reach the metallic state has resulted in researchers publishing before they can reproduce the results. Reproducibility is vital not only within one’s own research group, but also with others. The “Wigner–Huntington metallic hydrogen phase” discovery was announced more than three years ago,46 but no confirmation of the metallicity or of any other statements made in the paper, including pressures, has followed, either from other competing groups or, even more importantly, from the authors themselves. The lack of reproducibility also leads to inconsistencies in the literature: the same authors who claimed the existence of “atomic liquid metallic hydrogen” at 260 GPa in 201133 recently announced semimetallic solid hydrogen at above 400 GPa,22 leaving readers guessing as to which discovery to believe. The paper’s citations also create the impression that all recent important discoveries were made by the authors.22
Another example of a preferred interpretation of results was presented recently in the latest claim of “a first order phase transition to metal hydrogen near 425 GPa.”54 The authors present infrared absorption measurements (which by themselves are not enough to claim the existence of a metallic state) demonstrating that the amount of light going through a hydrogen sample at 425 GPa is reduced by a factor of around 10−2 compared with lower pressure. In their earlier paper,30 where metallization was not claimed, the same authors stated that “less than 2 × 10−3 of the visible white light was going through” the sample. When the arXiv manuscript54 came out in Nature,55 the authors diluted the claim of metallization by including the words “probable transition.” More interestingly, in both versions,54,55 the claims of hydrogen being semimetallic, as measured by directly probing the resistance of the sample,22 is swept under the carpet as “remain unconfirmed.” It is not clear why the dubious transmission experiment is more reliable and confirming, while the direct electrical measurement, which contradicts the claim of metallicity, is not. A serious paper should include an analysis of previous results if they happen to contradict one’s claim. An excellent example of such an approach is presented in Ref. 15, where the authors analyze the discrepancy between their own results and those of Ref. 14, obtained under essentially the same P–T conditions, thus providing an alternative explanation and interpretation. Such an analysis and comparison with other results is only possible when other researchers’ data are at least acknowledged to exist, which seems not to be the case in the static high-pressure hydrogen community.
It is clear that we are tantalizingly close to reaching the solid metallic state of hydrogen, but the reproducibility of results will require high-pressure techniques to develop to the point where we can convincingly reach pressures above 400 GPa, while still allowing a suite of diagnostics. Only when we have conclusively reached the solid metallic state of hydrogen can Ginzburg’s third “especially important and interesting” problem in physics be struck off the list.
Eugene Gregoryanz, Cheng Ji, Philip Dalladay-Simpson, Bing Li, Ross T. Howie, Ho-Kwang Mao. Everything you always wanted to know about metallic hydrogen but were afraid to ask[J]. Matter and Radiation at Extremes, 2020, 5(3): 038101.
 Download: 533次
Download: 533次