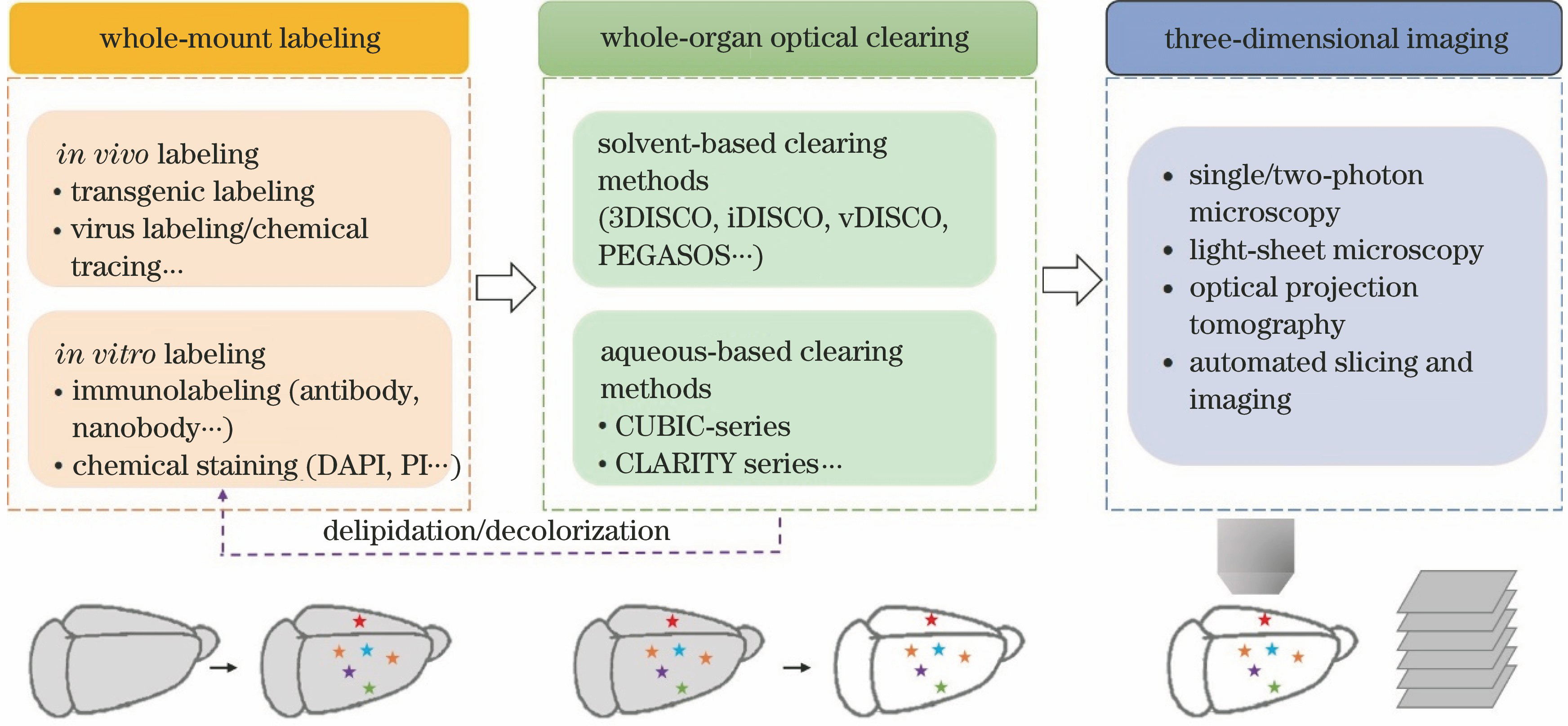
整体器官的光透明成像方法综述  下载: 2894次特邀综述
下载: 2894次特邀综述
俞婷婷, 朱丹. 整体器官的光透明成像方法综述[J]. 中国激光, 2020, 47(2): 0207007.
Yu Tingting, Zhu Dan. Review of Tissue Optical Clearing Methods for Imaging Whole Organs[J]. Chinese Journal of Lasers, 2020, 47(2): 0207007.
[1] Kim S Y, Chung K, Deisseroth K. Light microscopy mapping of connections in the intact brain[J]. Trends in Cognitive Sciences, 2013, 17(12): 596-599.
[2] Richardson D S, Lichtman J W. Clarifying tissue clearing[J]. Cell, 2015, 162(2): 246-257.
[3] Susaki E A, Ueda H R. Whole-body and whole-organ clearing and imaging techniques with single-cell resolution: toward organism-level systems biology in mammals[J]. Cell Chemical Biology, 2016, 23(1): 137-157.
[4] 骆清铭. 脑空间信息学——连接脑科学与类脑人工智能的桥梁[J]. 中国科学: 生命科学, 2017, 47(10): 1015-1024.
Luo Q M. Brainsmatics-bridging the brain science and brain-inspired artificial intelligence[J]. Scientia Sinica(Vitae), 2017, 47(10): 1015-1024.
[5] Zipfel W R, Williams R M, Webb W W. Nonlinear magic: multiphoton microscopy in the biosciences[J]. Nature Biotechnology, 2003, 21(11): 1369-1377.
[6] Conchello J A, Lichtman J W. Optical sectioning microscopy[J]. Nature Methods, 2005, 2(12): 920-931.
[7] Helmchen F, Denk W. Deep tissue two-photon microscopy[J]. Nature Methods, 2005, 2(12): 932-940.
[8] Huisken J, Swoger J, Del Bene F, et al. Optical sectioning deep inside live embryos by selective plane illumination microscopy[J]. Science, 2004, 305(5686): 1007-1009.
[9] Verveer P J, Swoger J, Pampaloni F, et al. High-resolution three-dimensional imaging of large specimens with light sheet-based microscopy[J]. Nature Methods, 2007, 4(4): 311-313.
[10] Chen B C, Legant W R, Wang K, et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution[J]. Science, 2014, 346(6208): 1257998.
[11] Stefaniuk M, Gualda E J, Pawlowska M, et al. Light-sheet microscopy imaging of a whole cleared rat brain with Thy1-GFP transgene[J]. Scientific Reports, 2016, 6: 28209.
[12] Voigt F F, Kirschenbaum D, Platonova E, et al. The mesoSPIM initiative: open-source light-sheet microscopes for imaging cleared tissue[J]. Nature Methods, 2019, 16(11): 1105-1108.
[13] Tuchin V V. Light propagation in tissues with controlled optical properties[J]. Journal of Biomedical Optics, 1997, 2(4): 401-417.
[14] Tuchin V V. Optical clearing of tissues and blood using the immersion method[J]. Journal of Physics D: Applied Physics, 2005, 38(15): 2497-2518.
[15] Tuchin VV. Optical clearing of tissues and blood[M]. Bellingham: SPIE, 2005.
[16] Genina E A, Bashkatov A N, Tuchin V V. Tissue optical immersion clearing[J]. Expert Review of Medical Devices, 2010, 7(6): 825-842.
[18] Wang T, Ouzounov D G, Wu C Y, et al. Three-photon imaging of mouse brain structure and function through the intact skull[J]. Nature Methods, 2018, 15(10): 789-792.
[19] Li J B, Liu H W, Fu T, et al. Recent progress in small-molecule near-IR probes for bioimaging[J]. Trends in Chemistry, 2019, 1(2): 224-234.
[22] Miyamichi K, Amat F, Moussavi F, et al. Cortical representations of olfactory input by trans-synaptic tracing[J]. Nature, 2011, 472(7342): 191-196.
[23] Ertürk A, Bradke F. High-resolution imaging of entire organs by 3-dimensional imaging of solvent cleared organs (3DISCO)[J]. Experimental Neurology, 2013, 242: 57-64.
[25] Li A, Gong H, Zhang B, et al. Micro-optical sectioning tomography to obtain a high-resolution atlas of the mouse brain[J]. Science, 2010, 330(6009): 1404-1408.
[26] Gong H, Xu D L, Yuan J, et al. High-throughput dual-colour precision imaging for brain-wide connectome with cytoarchitectonic landmarks at the cellular level[J]. Nature Communications, 2016, 7: 12142.
[27] Luo Y L, Wang A L, Liu M M, et al. Label-free brainwide visualization of senile plaque using cryo-micro-optical sectioning tomography[J]. Optics Letters, 2017, 42(21): 4247-4250.
[28] Zhu D, Larin K V, Luo Q M, et al. Recent progress in tissue optical clearing[J]. Laser & Photonics Reviews, 2013, 7(5): 732-757.
[29] Yu T T, Qi Y S, Gong H, et al. Optical clearing for multiscale biological tissues[J]. Journal of Biophotonics, 2018, 11(2): e201700187.
[30] Vigouroux R J, Belle M, Chédotal A. Neuroscience in the third dimension: shedding new light on the brain with tissue clearing[J]. Molecular Brain, 2017, 10: 33.
[31] Miyawaki A. Brain clearing for connectomics[J]. Microscopy, 2015, 64(1): 5-8.
[32] Tainaka K, Kuno A, Kubota S I, et al. Chemical principles in tissue clearing and staining protocols for whole-body cell profiling[J]. Annual Review of Cell and Developmental Biology, 2016, 32(1): 713-741.
[33] Silvestri L, Costantini I, Sacconi L, et al. Clearing of fixed tissue: a review from a microscopist's perspective[J]. Journal of Biomedical Optics, 2016, 21(8): 081205.
[35] Chung K, Wallace J. KimS Y, et al. Structural and molecular interrogation of intact biological systems[J]. Nature, 2013, 497(7449): 332-337.
[36] Jing D, Zhang S W, Luo W J, et al. Tissue clearing of both hard and soft tissue organs with the PEGASOS method[J]. Cell Research, 2018, 28(8): 803-818.
[37] TainakaK, Murakami TC, Susaki EA, et al., 2018, 24(8): 2196-2210. e9.
[38] Moffitt J R, Hao J J, Bambah-Mukku D, et al. High-performance multiplexed fluorescence in situ hybridization in culture and tissue with matrix imprinting and clearing[J]. Proceedings of the National Academy of Sciences, 2016, 113(50): 14456-14461.
[41] Kim S Y, Cho J H, Murray E, et al. Stochastic electrotransport selectively enhances the transport of highly electromobile molecules[J]. Proceedings of the National Academy of Sciences, 2015, 112(46): E6274-E6283.
[42] Lee E, Choi J, Jo Y, et al. ACT-PRESTO: rapid and consistent tissue clearing and labeling method for 3-dimensional (3D) imaging[J]. Scientific Reports, 2016, 6: 18631.
[43] Greenbaum A, ChanK Y, Dobreva T, et al. 9(387): eaah6518[J]. imaging, computational analysis of osteoprogenitors within intact bone marrow. Science Translational Medicine, 2017.
[44] Zhao Y J, Yu T T, Zhang C, et al. Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution[J]. Light: Science & Applications, 2018, 7(2): 17153.
[45] Zhang C, Feng W, Zhao Y J, et al. A large, switchable optical clearing skull window for cerebrovascular imaging[J]. Theranostics, 2018, 8(10): 2696-2708.
[47] Kristinsson H G, Hultin H O. Changes in trout hemoglobin conformations and solubility after exposure to acid and alkali pH[J]. Journal of Agricultural and Food Chemistry, 2004, 52(11): 3633-3643.
[48] Treweek J B, Chan K Y, Flytzanis N C, et al. Whole-body tissue stabilization and selective extractions via tissue-hydrogel hybrids for high-resolution intact circuit mapping and phenotyping[J]. Nature Protocols, 2015, 10(11): 1860-1896.
[49] Susaki E A, Tainaka K, Perrin D, et al. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging[J]. Nature Protocols, 2015, 10(11): 1709-1727.
[50] Kubota S I, Takahashi K, Nishida J, et al. Whole-body profiling of cancer metastasis with single-cell resolution[J]. Cell Reports, 2017, 20(1): 236-250.
[51] Ke M T, Fujimoto S, Imai T. See DB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction[J]. Nature Neuroscience, 2013, 16(8): 1154-1161.
[52] Murray E. ChoJ H, Goodwin D, et al. Simple, scalable proteomic imaging for high-dimensional profiling of intact systems[J]. Cell, 2015, 163(6): 1500-1514.
[53] Tsai P S, Kaufhold J P, Blinder P, et al. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels[J]. The Journal of Neuroscience, 2009, 29(46): 14553-14570.
[54] Hou B, Zhang D, Zhao S, et al. Scalable and DiI-compatible optical clearance of the mammalian brain[J]. Frontiers in Neuroanatomy, 2015, 9: 19.
[55] Economo M N, Clack N G, Lavis L D, et al. A platform for brain-wide imaging and reconstruction of individual neurons[J]. eLife, 2016, 5: e10566.
[57] Lai H M. Liu A K L, Ng H H M, et al. Next generation histology methods for three-dimensional imaging of fresh and archival human brain tissues[J]. Nature Communications, 2018, 9: 1066.
[58] Chen L L, Li G Y, Li Y M, et al. Ubas M: an effective balanced optical clearing method for intact biomedical imaging[J]. Scientific Reports, 2017, 7: 12218.
[60] Ke M T, Nakai Y, Fujimoto S, et al. Super-resolution mapping of neuronal circuitry with an index-optimized clearing agent[J]. Cell Reports, 2016, 14(11): 2718-2732.
[61] Li W Z, Germain R N, Gerner M Y. Multiplex, quantitative cellular analysis in large tissue volumes with clearing-enhanced 3D microscopy (Ce3D)[J]. Proceedings of the National Academy of Sciences, 2017, 114(35): E7321-E7330.
[62] Ariel P. A beginner's guide to tissue clearing[J]. The International Journal of Biochemistry & Cell Biology, 2017, 84: 35-39.
[63] Becker K, Jährling N, Kramer E R, et al. Ultramicroscopy: 3D reconstruction of large microscopical specimens[J]. Journal of Biophotonics, 2008, 1(1): 36-42.
[64] Dodt H U, Leischner U, Schierloh A, et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain[J]. Nature Methods, 2007, 4(4): 331-336.
[65] Jährling N, Becker K, Dodt H U. 3D-reconstruction of blood vessels by ultramicroscopy[J]. Organogenesis, 2009, 5(4): 227-230.
[66] Jährling N. Three-dimensional reconstruction and segmentation of intact Drosophila by ultramicroscopy[J]. Frontiers in System Neuroscience, 2010, 4: 1.
[67] Becker K, Jährling N, Saghafi S, et al. Chemical clearing and dehydration of GFP expressing mouse brains[J]. PLoS One, 2012, 7(3): e33916.
[69] Schwarz M K, Scherbarth A, Sprengel R, et al. Fluorescent-protein stabilization and high-resolution imaging of cleared, intact mouse brains[J]. PLoS One, 2015, 10(5): e0124650.
[70] Klingberg A, Hasenberg A, Ludwig-Portugall I, et al. Fully automated evaluation of total glomerular number and capillary tuft size in nephritic kidneys using lightsheet microscopy[J]. Journal of the American Society of Nephrology, 2017, 28(2): 452-459.
[71] MasselinkW, ReumannD, MurawalaP, et al., 2019, 146(3): dev166884.
[72] Pan C C, Cai R Y, Quacquarelli F P, et al. Shrinkage-mediated imaging of entire organs and organisms using uDISCO[J]. Nature Methods, 2016, 13(10): 859-867.
[73] Belle M, Godefroy D, Couly G, et al. 169(1): 161-[J]. analysis of early human development. Cell, 2017, 173: e12.
[74] Renier N, Adams E L, Kirst C, et al. Mapping of brain activity by automated volume analysis of immediate early genes[J]. Cell, 2016, 165(7): 1789-1802.
[75] Wang Q, Liu K L, Yang L, et al. BoneClear: whole-tissue immunolabeling of the intact mouse bones for 3D imaging of neural anatomy and pathology[J]. Cell Research, 2019, 29(10): 870-872.
[76] Cai R Y, Pan C C, Ghasemigharagoz A, et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections[J]. Nature Neuroscience, 2019, 22(2): 317-327.
[77] Hildebrand S, Schueth A, Herrler A, et al. Scalable labeling for cytoarchitectonic characterization of large optically cleared human neocortex samples[J]. Scientific Reports, 2019, 9: 10880.
[78] Hama H, Kurokawa H, Kawano H, et al. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain[J]. Nature Neuroscience, 2011, 14(11): 1481-1488.
[79] Hama H, Hioki H, Namiki K, et al. ScaleS: an optical clearing palette for biological imaging[J]. Nature Neuroscience, 2015, 18(10): 1518-1529.
[80] Murakami T C, Mano T, Saikawa S, et al. A three-dimensional single-cell-resolution whole-brain atlas using CUBIC-X expansion microscopy and tissue clearing[J]. Nature Neuroscience, 2018, 21(4): 625-637.
[82] Epp JR, NiiboriY, ( Liz) Hsiang HL, et al. and other intact organs[J].Eneuro, 2015, 2(3): ENEURO. 0022-15. 2015.
[83] Bastrup J, Larsen P H. Optimized CLARITY technique detects reduced parvalbumin density in a genetic model of schizophrenia[J]. Journal of Neuroscience Methods, 2017, 283: 23-32.
[84] Ren J, Choi H, Chung K, et al. Label-free volumetric optical imaging of intact murine brains[J]. Scientific Reports, 2017, 7: 46306.
[85] Ku T, Swaney J, Park J Y, et al. Multiplexed and scalable super-resolution imaging of three-dimensional protein localization in size-adjustable tissues[J]. Nature Biotechnology, 2016, 34(9): 973-981.
[86] Park Y G, Sohn C H, Chen R, et al. Protection of tissue physicochemical properties using polyfunctional crosslinkers[J]. Nature Biotechnology, 2019, 37(1): 73-83.
[88] Sylwestrak E L, Rajasethupathy P, Wright M A, et al. Multiplexed intact-tissue transcriptional analysis at cellular resolution[J]. Cell, 2016, 164(4): 792-804.
[89] Qi YS, Yu TT, Xu JY, et al., 2019, 5(1): eaau8355.
[91] Diekmann H, Kalbhen P, Fischer D. Characterization of optic nerve regeneration using transgenic zebrafish[J]. Frontiers in Cellular Neuroscience, 2015, 9: 118.
[92] Frétaud M, Rivière L, De Job É, et al. High-resolution 3D imaging of whole organ after clearing: taking a new look at the zebrafish testis[J]. Scientific Reports, 2017, 7: 43012.
[93] Hsiao P Y, Tsai C L, Chen M C, et al. Non-invasive manipulation of Drosophila behavior by two-photon excited red-activatable channelrhodopsin[J]. Biomedical Optics Express, 2015, 6(11): 4344-4352.
[94] Žygelyté E, Bernard M E, Tomlinson J E, et al. RetroDISCO: clearing technique to improve quantification of retrograde labeled motor neurons of intact mouse spinal cords[J]. Journal of Neuroscience Methods, 2016, 271: 34-42.
[95] Liu A K L, Hurry M E D, Ng O T W, et al. Bringing CLARITY to the human brain: visualization of Lewy pathology in three dimensions[J]. Neuropathology and Applied Neurobiology, 2016, 42(6): 573-587.
[96] Grist S M, Nasseri S S, Poon T, et al. On-chip clearing of arrays of 3-D cell cultures and micro-tissues[J]. Biomicrofluidics, 2016, 10(4): 044107.
[97] Silva Santisteban T, Rabajania O, Kalinina I, et al. Rapid spheroid clearing on a microfluidic chip[J]. Lab on a Chip, 2018, 18(1): 153-161.
[98] Chen Y Y, Silva P N, Syed A M, et al. Clarifying intact 3D tissues on a microfluidic chip for high-throughput structural analysis[J]. Proceedings of the National Academy of Sciences, 2016, 113(52): 14915-14920.
[99] Zhang Y B, Shin Y, Sung K, et al. 3D imaging of optically cleared tissue using a simplified CLARITY method and on-chip microscopy[J]. Science Advances, 2017, 3(8): e1700553.
[100] Liebmann T, Renier N, Bettayeb K, et al. Three-dimensional study of alzheimer's disease hallmarks using the iDISCO clearing method[J]. Cell Reports, 2016, 16(4): 1138-1152.
[101] Kim SH, CheP, Chung SH, et al., 2006, 36(1): 2. 10.1-2.10. 9.
[103] Gleave J A, Lerch J P, Henkelman R M, et al. A method for 3D immunostaining and optical imaging of the mouse brain demonstrated in neural progenitor cells[J]. PLoS One, 2013, 8(8): e72039.
[104] Seo J, Choe M, Kim S Y. Clearing and labeling techniques for large-scale biological tissues[J]. Molecules and Cells, 2016, 39(6): 439-446.
[105] Li J, Czajkowsky D M, Li X W, et al. Fast immuno-labeling by electrophoretically driven infiltration for intact tissue imaging[J]. Scientific Reports, 2015, 5: 10640.
[107] GaoR, Asano SM, UpadhyayulaS, et al. and whole-brain imaging with molecular contrast and nanoscale resolution[J].Science, 2019, 363(6424): eaau8302.
[108] Keller P J, Schmidt A D, Wittbrodt J, et al. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy[J]. Science, 2008, 322(5904): 1065-1069.
[109] Tomer R, Lovett-Barron M, Kauvar I, et al. SPED light sheet microscopy: fast mapping of biological system structure and function[J]. Cell, 2015, 163(7): 1796-1806.
[110] Pende M, Becker K, Wanis M, et al. High-resolution ultramicroscopy of the developing and adult nervous system in optically cleared Drosophila melanogaster[J]. Nature Communications, 2018, 9: 4731.
[112] Wang H, Zhu Q Y, Ding L F, et al. Scalable volumetric imaging for ultrahigh-speed brain mapping at synaptic resolution[J]. National Science Review, 2019, 6(5): 982-992.
[113] Costantini I. Ghobril J P, di Giovanna A P, et al. A versatile clearing agent for multi-modal brain imaging[J]. Scientific Reports, 2015, 5: 9808.
[116] Sharpe J, Ahlgren U, Perry P, et al. Optical projection tomography as a tool for 3D microscopy and gene expression studies[J]. Science, 2002, 296(5567): 541-545.
[117] Osten P, Margrie T W. Mapping brain circuitry with a light microscope[J]. Nature Methods, 2013, 10(6): 515-523.
[118] Oldham M, Sakhalkar H, Oliver T, et al. Optical clearing of unsectioned specimens for three-dimensional imaging via optical transmission and emission tomography[J]. Journal of Biomedical Optics, 2008, 13(2): 021113.
俞婷婷, 朱丹. 整体器官的光透明成像方法综述[J]. 中国激光, 2020, 47(2): 0207007. Yu Tingting, Zhu Dan. Review of Tissue Optical Clearing Methods for Imaging Whole Organs[J]. Chinese Journal of Lasers, 2020, 47(2): 0207007.