Nowadays, luminescent optical fibers are an attractive research topic. Among numerous applications of glass and polymer-based optical fibers are optical amplifiers, lasers, light sources, sensors, and scintillators[1–9" target="_self" style="display: inline;">–9]. The polymeric optical fibers (POFs) offer some advantages over silica ones (high aperture and core diameter, easy coupling to cooperating devices, flexibility, easy processing, the possibility of doping by organic and inorganic compounds). In such circumstances, the new advanced organic-host-based materials can be applied in optical fiber technology. The luminescent POFs doped with organic dyes are well-known from their bright luminescence, since numerous fluorescent dyes offer high absorption and emission cross sections. The most frequently used are based on xanthene, amine, and oxazine groups[10–13" target="_self" style="display: inline;">–13]. In fact, the main disadvantage of organic dyes is the photobleaching phenomenon, which significantly limits their commercial applications. The organic dye’s solvent-based laser wavelength converters are well-known. This idea can be applied to rigid polymer optical fibers. According to the fast depopulation of the excited state [typically few nanoseconds (ns)] of organic fluorescent dyes and the photobleaching effect, the laser action in POFs can be obtained for the pulsed regime. Much longer lifetimes of the excited state reported for lanthanides [typically a few milliseconds (ms)] allows for potential applications in the continuous regime operation in fiber lasers and amplifiers[1416" target="_self" style="display: inline;">–16]. The well-defined luminescence spectrum shape of trivalent lanthanide ions is useful in numerous applications. Unfortunately, their absorption spectra are very narrow, and effective excitation is limited to a few main wavelengths. This problem can be reduced by using the organometallic structure. The additional organic ligand allows the shielding of the lanthanide ion from the polymeric host and acts as a wide absorption band antenna. The non-radiative energy transfer from excited organic molecules to rare earth ions significantly improves the excitation possibility. Taking into account the technological aspects, the organometallic compounds cannot be used for doping the inorganic-host-based fibers, since the temperatures of the drawing of soft glass (c.a., 300°C–800°C) and silica (1750°C–2100°C) are significantly higher than the organic ligand breakdown[17]. In such cases, the doping possibility is limited to polymer-based technology.
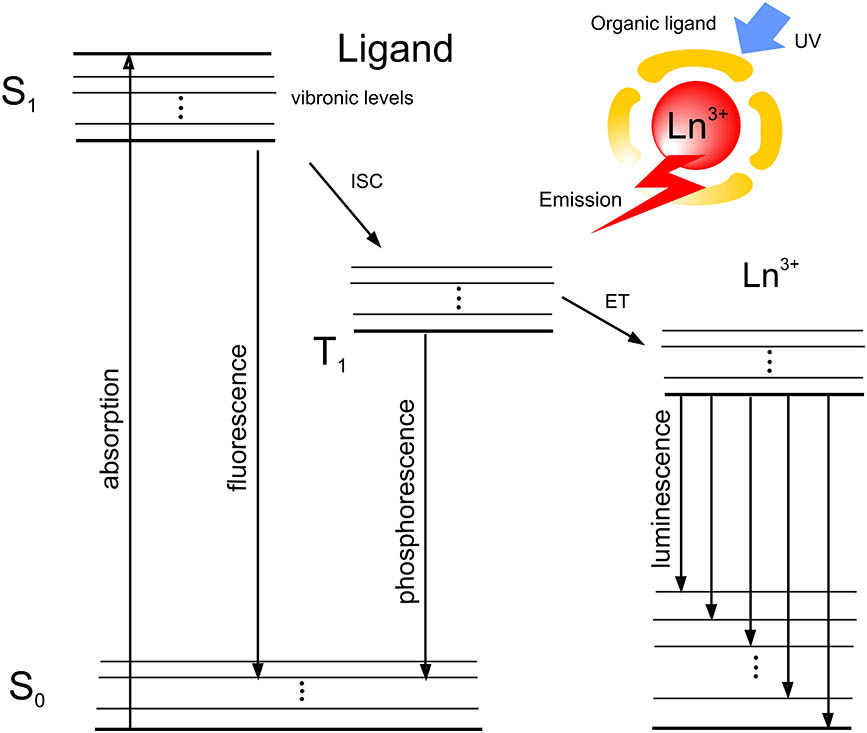
The incorporation of lanthanide ions in the polymeric host creates the competition between the radiative and non-radiative processes. Although luminescence quenching is evidence of the non-radiative transitions (multiphonon relaxation), the proper ligand choice allows energy transitions pathways, which promote luminescent transitions[18]. The typical energy state scheme is presented in Fig. 1.
Fig. 1. Simplified energy state scheme of the organometallic chelate. Insertion: antenna effect. UV, ultraviolet; ET, energy transfer; ISC, intersystem crossing.
下载图片 查看所有图片
The molecule of the ligand can be effectively excited due to its wide absorption band. Then, excited singlet state of the ligand can be deactivated through fluorescence or energy transfer to triplet state and then transferred to a rare earth ion or deactivated by phosphorescent radiation. The proper energy level of the triplet state allows for an efficient energy transfer to the excited state of the lanthanide ion and luminescence to the ground state. The well-known polymer for optoelectronic applications is poly(methyl methacrylate) (PMMA), which is called organic glass. High transparency and thermoplasticy assure excellent processability conditions for organometallic-doped optical fibers. Nowadays, developing the technology of hybrid organic–inorganic materials allows for obtaining new features of luminescent materials[19–23" target="_self" style="display: inline;">–23]. The waveguiding properties of optical fibers allow for significant modification of the luminescence spectrum versus fiber length. Numerous phenomena attend to luminescence spectrum shape modification (reabsorption, spectral attenuation, nonlinear effects, etc.). The well-known, so-called “red shift” phenomenon is often observed for fluorescent dyes-doped optical fibers, since the emission and absorption spectra overlap each other[24]. Opposite of fluorescent organic dyes, lanthanides assure a wide gap between the absorption and emission spectra. Therefore, the spectroscopic property’s characterization of POFs is crucial for new application development. This Letter presents the trivalent terbium-doped PMMA fiber fabrication process and spectrum shape modification in the polymeric fiber structure.
The fiber was fabricated at the Bialystok University of Technology laboratory. The methyl methacrylate (MMA), benzoyl peroxide (BP), butanethiol (Bl), and Terbium(III)-tris-(2,2,6,6-tetramethyl-3,5-heptanedionate) were supplied by Sigma-Aldrich with a standard purity. The stabilizer agent has been removed from the monomer before the polymerization process (free radical polymerization, 44 h at 65°C–80°C). The chelate doping process was performed directly during fiber preform fabrication (10 mm outer diameter, 0.6% w/w concentration). No polymerization defects (cracking or bubbles) were observed in the preform. The terbium-doped PMMA specimens were prepared by cutting and polishing 2.0 mm thick disks. The characterized fiber was fabricated using a computer controlled optical fiber tower (furnace temperatures , preform feeding 0.5 cm/min, drawing speed 20–32 cm/min). The fibers’ diameters ranging from 0.9–1.9 mm were produced. The fiber diameter chosen for characterization was 1.5 mm, since low excitation laser power density has to be used (glass transition temperature of PMMA is about 105°C). The luminescence of the terbium-doped PMMA fiber is presented in Fig. 2.
Fig. 2. Photo of the fabricated fiber under 355 nm with one end excitation. Insertion: -doped PMMA specimen.
下载图片 查看所有图片
The excitation bulk samples were characterized in terms of excitation and emission spectra and excited state lifetime. The excitation and emission spectra of the bulk specimen were measured using a xenon lamp (450 W) and a fluorescence spectrometer (Horiba Fluorolog 3). The lifetime measurements were performed at a third harmonic (355 nm) of an Nd:YAG laser. The luminescence of the terbium-doped fiber was measured using the cutback method. The collimator was used for the efficient coupling of laser radiation into the fiber. The spectral attenuation was measured using a halogen Stellarnet SL1 lamp. All luminescence spectra were recorded using a Stellarnet Green Wave spectrometer (400–900 nm). The measurements have been performed at a temperature of 20°C.
The measured excitation spectrum [Fig. 3(a)] is a composition of direct terbium absorption bands, according to (378 nm), (486 nm), and a wide peak at . The excitation spectrum shape confirms efficient excitation of the terbium complex by ligand (antenna effect) and direct rare earth ions. The identified energy state transitions are marked on the luminescence spectrum measured at [Fig. 3(b)].
Fig. 3. -doped PMMA luminescence spectra: (a) excitation spectrum measured at a monitoring of 542 nm and (b) emission spectrum at an excitation of 355 nm.
下载图片 查看所有图片
The luminescence of lanthanide ions due to the lanthanide ion environment is limited to a few main peaks. However, high-intensity peaks (486 nm), (547 nm), (582 nm), (629 nm), and (655 nm) luminescent peaks are clearly noticeable. The recorded spectra are similar for those reported in glass matrices. The main emission peak (547 nm) in the terbium structure can be used for ion environment characterization in some organometallic complexes[25–29" target="_self" style="display: inline;">–29]. The energy states transition leads to the Stark-split doublet with luminescence peaks at 543 and 547 nm ()[3032" target="_self" style="display: inline;">–32]. Moreover, no broadband ligand luminescence peak can be observed in the recorded spectrum, since the energy transfer from the ligand triplet state to of the terbium level can occur. However, the single excited state can also directly transfer the energy to higher energy levels of the lanthanide ion and then to the level by non-radiative relaxations. The luminescence decay curve of the excited state (Fig. 4) was measured at a 355 nm excitation and transition (542 nm). The single phase decay curve (adjusted ) can be used for decay character estimation. An intense luminescence and relatively long measured excited state () lifetime of 0.741 ms confirm that the used terbium organometallic structure can be successfully applied to PMMA doping. The obtained results are in good agreement with what was reported in Refs. [33,34].
Fig. 4. Luminescence decay time curve at an excitation of 355 nm and monitoring at 542 nm.
下载图片 查看所有图片
The fiber luminescent property’s characterization was performed using a 355 nm excitation wavelength. The spectra presented in Fig. 5(a) show characteristic luminescence peaks observed in the bulk specimen. Opposite of organic-dyes-doped polymeric fibers, no spectrum shift versus fiber length can be observed in the recorded spectra. The lanthanide ions’ absorption and emission spectra are separated, and no reabsorption effect can be observed in the luminescence spectra. It is noticeable that the luminescence spectrum significantly changes versus fiber length. The normalized spectra to the main transition show that the peak corresponds to transition decrease over the distance of 70 cm. The much higher peak’s content is observed for a distance of 70 cm for the transitions (582 nm), (629 nm), and (655 nm) than for 5 cm. The pump radiation absorption significantly decreases the pump intensity (pump radiation was totally absorbed over the distance of 5 cm). It is related to the high absorption cross section of the used ligand and host attenuation. The noticed spectrum shape modification is in good agreement with the measured spectral attenuation of the fabricated fiber [Fig. 5(b)]. The relatively high attenuation level is noticeable due to the fiber construction (air cladding optical fiber structure) and host attenuation (PMMA). The minimum of attenuation of 15 dB/m PMMA occurs for 845 nm. The PMMA structure causes Rayleigh scattering for short wavelengths and molecular absorption for longer wavelengths of the optical spectrum. The minimum attenuation of undoped PMMA is typically observed for the visible (VIS) spectrum range.
Fig. 5. (a) Normalized luminescence spectrum of a terbium-doped PMMA fiber, (b) spectral attenuation of trivalent terbium-compounds-doped PMMA fibers, and (c) the ratio of the main peaks versus the fiber length.
下载图片 查看所有图片
The intensity peaks ratio (A) and (B) to the main transition are presented in Fig. 5(c). The ratio increases in the range of 0.13–0.35 () and 0.08–0.45 (). The observed spectrum modification corresponds to the measured attenuation (43 dB), (34 dB), and (32 dB). Significantly higher attenuation of the main transition peak (at 547 nm) gives the possibility of spectral shape modifications in the final optical fiber structure. The chelating structure of terbium embedded in the polymeric fiber allows for obtaining an intense luminescence signal. The length of most of the luminescent polymer-based optical fibers’ constructions is limited due to the fact of high attenuation. For some applications, the spectral attenuation can be useful for optimization of the luminescence spectrum shape. Moreover, a new class of optical fiber amplifiers or lasers can be investigated on the basis of lanthanide-doped hybrid organic–inorganic materials. The presented results are important for new optical devices’ constructions based on POFs.
The terbium-chelate-based PMMA optical fiber characterization is presented. An intense luminescence of trivalent terbium in PMMA is presented. The measured excitation state lifetime of 0.741 ms confirms low deactivation of the excited state by non-radiative processes. The luminescence spectrum shape modification versus the fiber length under 355 nm allows for a modification of luminescence, according to spectral attenuation of the polymeric fiber. The peak’s ratio increases in the range 0.13–0.35 () and 0.08–0.45 (). The presented terbium chelate optical fiber exhibits the opportunity to advance new light sources, amplifiers, lasers, and sensors constructions.
P. Miluski, M. Kochanowicz, J. Zmojda, D. Dorosz. Luminescent properties of Tb3+-doped poly(methyl methacrylate) fiber[J]. Chinese Optics Letters, 2017, 15(7): 070602.