碱土金属离子共掺杂对铕离子在Ca12Al14O32F2中发光性能的影响  下载: 625次
下载: 625次
1 引言
稀土铕离子具有丰富的电子能级,常被用作荧光粉的激活剂[1-2]。在铕离子的2种价态中,Eu3+的f➝f禁阻跃迁呈尖峰发射[3-4],而Eu2+的d➝f跃迁呈宽峰发射[5-6]。在单一荧光基质中,若能使Eu3+和Eu2+共存,或使Eu3+较多地转化为Eu2+,就可调整Eu3+的红光发射与Eu2+的蓝光发射,从而有可能得到蓝白光荧光粉,甚至得到白光荧光粉。近年来,不少文献报道了Eu3+和Eu2+的共存体系,如磷酸盐、硼酸盐、硅酸盐和铝酸盐[1,2,7-13],但对氟铝酸盐体系的报道还很少[14-15]。氟铝酸盐Ca12Al14O32F2为立方晶体结构,具有透光范围宽、折射率低和化学性能稳定等特点,常被用作荧光粉的基质[14-17]。Huang等[14]采用高温固相法在还原氛围下合成了Ca12Al14O32F2∶Eu荧光粉,通过掺杂少量硅离子,使Si4+-O2-部分取代Al3+-F-,从而使Eu3+还原为Eu2+。陈彩花等[15]采用燃烧法在空气氛围下合成Ca12Al14O32F2∶Eu荧光粉,还原剂尿素和氟化铵将一部分Eu3+还原为Eu2+。Peng等[16]采用溶胶凝胶法在空气氛围下合成Ca12Al14O32F2∶Eu时[16],主要得到了Eu3+掺杂的荧光粉,但发射光谱中出现了极微弱的Eu2+发射峰,这应该是干胶在热处理过程中产生的还原气氛造成的。本文利用高温固相还原法在Ca12Al14O32F2∶Eu体系中掺杂少量碱土金属离子(Mg, Sr, Ba),通过改变碱土金属离子的掺杂浓度改变激活离子存在的晶胞局部环境,进而调节Eu3+和Eu2+的浓度比,最终达到调节蓝光发射和红光发射的强度比。目前,已有文献报道了碱土金属离子的尺寸效应对
2 实验部分
根据Ca12-
采用XD-3型X射线衍射仪(XRD)测试目标产物的物相,铜靶(Kα射线,
3 结果与讨论
Ca12Al14O32F2为立方晶系结构[14,17],空间群为
表 1. Ca2+配位数为7时各金属离子的半径及相对半径
Table 1. Radius and relative radius of metal ions when the coordination number of Ca2+ is 7
|
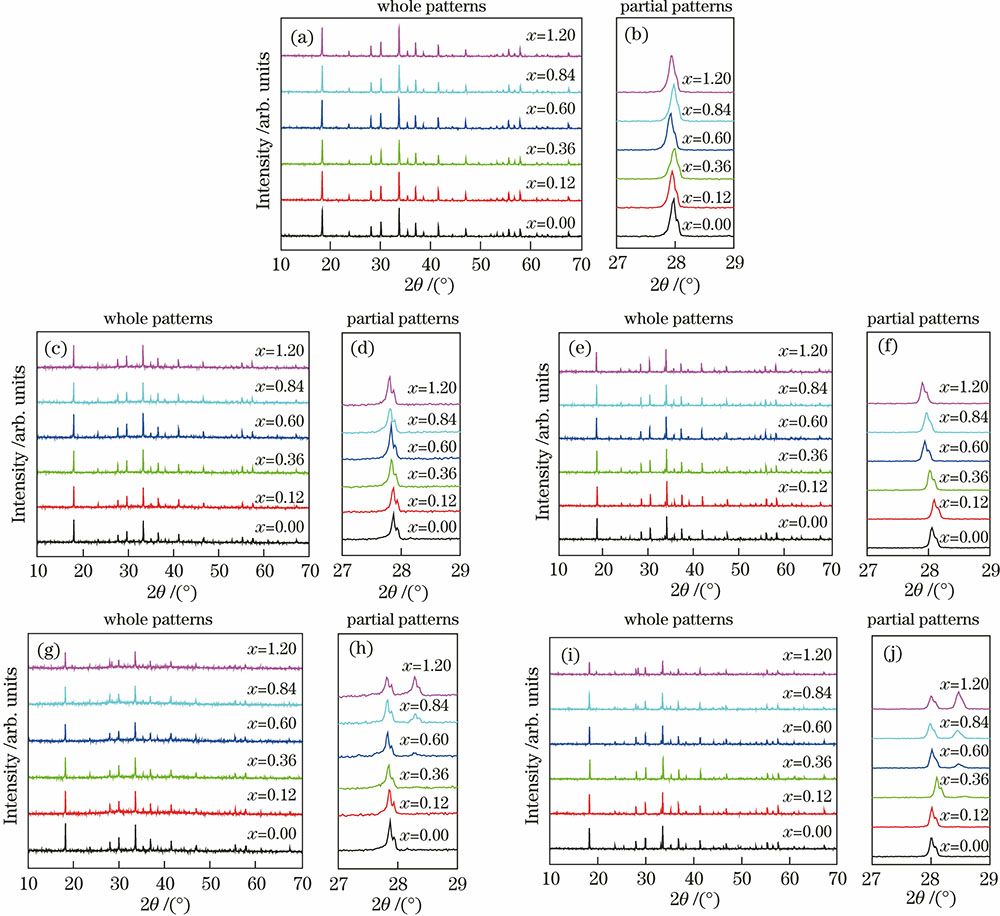
图 1. Ca12-x-yMxAl14O32F2∶yEu的XRD图谱。(a)(b) M=Mg, y=0.10; (c)(d) M=Sr, y=0.10; (e)(f) M=Sr, y=0.48; (g)(h) M=Ba,y=0.10; (i)(j) M=Ba, y=0.48
Fig. 1. XRD patterns of Ca12-x-yMxAl14O32F2∶yEu. (a)(b) M=Mg, y=0.10; (c)(d) M=Sr, y=0.10; (e)(f) M=Sr, y=0.48; (g)(h) M=Ba, y=0.10; (i)(j) M=Ba, y=0.48
铕离子及碱金属离子少量掺杂取代Ca2+对衍射峰角度的影响同样反映在晶胞参数上,Ca12-
表 2. Ca12-x-yMxAl14O32F2∶yEu的晶胞参数
Table 2. Cell parameters of Ca12-x-yMxAl14O32F2∶yEu
|

图 2. Ca11.90-xMgxAl14O32F2∶0.10Eu(x=1.20)的激发光谱和发射光谱
Fig. 2. Excitation and emission spectra of Ca11.90-xMgxAl14O32F2∶0.10Eu (x=1.20)

图 3. Ca11.90-xMgxAl14O32F2∶0.10Eu的(a)~(c)发射光谱及对应的(d)CIE色度坐标图
Fig. 3. (a)-(c) Emission spectra of Ca11.90-xMgxAl14O32F2∶0.10Eu and (d) CIE chromaticity diagram
增大,蓝光发射强度降低,红光发射强度增强。

图 4. (a)(b) Ca11.90-xSrxAl14O32F2∶0.10Eu的发射光谱;(c) Ca11.52-xSrxAl14O32F2∶0.48Eu的发射光谱;(d) Ca12-x-ySrxAl14O32F2∶yEu(y=0.10, 0.48)发射光谱对应的CIE色度坐标图
Fig. 4. (a)(b) Emission spectra of Ca11.90-xSrxAl14O32F2∶0.10Eu; (c) emission spectra of Ca11.52-xSrxAl14O32F2∶0.48Eu emission spectra; (d) CIE chromaticity diagrams of Ca12-x-ySrxAl14O32F2∶yEu (y=0.10, 0.48)

图 5. (a)~(c) Ca11.90-xBaxAl14O32F2∶0.10Eu的发射光谱;(d) Ca11.90-xBaxAl14O32F2∶0.10Eu的CIE色度坐标图;(e) Ca11.52-xBaxAl14O32F2∶0.48Eu的发射光谱;(f) Ca11.52-xBaxAl14O32F2∶0.48Eu的CIE色度坐标图
Fig. 5. (a)-(c) Emission spectra of Ca11.90-xBaxAl14O32F2∶0.10Eu; (d) CIE chromaticity diagrams of Ca11.90-xBaxAl14O32F2∶0.10Eu; (e) Emission spectra of Ca11.52-xBaxAl14O32F2∶0.48Eu; (f) CIE chromaticity diagrams of Ca11.52-xBaxAl14O32F2∶0.48Eu
4 结论
利用高温固相还原法合成了Eu3+和Eu2+共存的Ca12Al14O32F2∶Eu发光材料,通过碱土金属离子掺杂改变基质组成,调控铕离子晶胞的局部环境,进而调节Eu3+和Eu2+的浓度比,最终达到调整蓝光发射和红光发射强度比的目的。结果表明,在Ca12Al14O32F2∶Eu样品中掺杂一定浓度的Mg2+不利于Eu3+的还原,掺杂一定浓度的Sr2+或Ba2+有利于Eu3+的还原。改变碱土金属离子的掺杂浓度可以调整Eu2+的蓝光发射和Eu3+的红光发射的强度比,进而使样品的发光颜色从蓝色变为淡紫色,再变为蓝绿色。
[1] Yu R J, Wang J, Zhao Z, et al. Structure and tunable blue-white-red luminescence of Eu 2+/Eu 3+-doped Na5Al(PO4)2F2 single-phase phosphor [J]. Materials Letters, 2015, 160: 294-297.
Yu R J, Wang J, Zhao Z, et al. Structure and tunable blue-white-red luminescence of Eu 2+/Eu 3+-doped Na5Al(PO4)2F2 single-phase phosphor [J]. Materials Letters, 2015, 160: 294-297.
[4] 王林香. 合成条件对(Eu0. 045Li3xLuy)2O3纳米晶发光性能的影响[J]. 光学学报, 2016, 36(3): 0316001.
王林香. 合成条件对(Eu0. 045Li3xLuy)2O3纳米晶发光性能的影响[J]. 光学学报, 2016, 36(3): 0316001.
[5] 游潘丽, 胡曰博. BaMgSiO4∶Eu 2+/Eu 3+可调白光发光材料的制备和性能研究 [J]. 光学学报, 2014, 34(5): 0516001.
游潘丽, 胡曰博. BaMgSiO4∶Eu 2+/Eu 3+可调白光发光材料的制备和性能研究 [J]. 光学学报, 2014, 34(5): 0516001.
[7] Hou JS, Jiang WZ, Fang YZ, et al. Red, green and blue emissions coexistence in white-light-emitting Ca11( SiO4)4, 2013( 37): 5892- 5898.
Hou JS, Jiang WZ, Fang YZ, et al. Red, green and blue emissions coexistence in white-light-emitting Ca11( SiO4)4, 2013( 37): 5892- 5898.
[9] Sokolnicki J, Zych E. Synthesis and spectroscopic investigations of Sr2Y8(SiO4)6O2∶Eu 2+, Eu 3+ phosphor for white LEDs [J]. Journal of Luminescence, 2015, 158: 65-69.
Sokolnicki J, Zych E. Synthesis and spectroscopic investigations of Sr2Y8(SiO4)6O2∶Eu 2+, Eu 3+ phosphor for white LEDs [J]. Journal of Luminescence, 2015, 158: 65-69.
[11] Dobrowolska A, Zych E. Spectroscopic characterization of Ca3Y2Si3O12∶Eu 2+, Eu 3+ powders in VUV-UV-vis region [J]. The Journal of Physical Chemistry C, 2012, 116(48): 25493-25503.
Dobrowolska A, Zych E. Spectroscopic characterization of Ca3Y2Si3O12∶Eu 2+, Eu 3+ powders in VUV-UV-vis region [J]. The Journal of Physical Chemistry C, 2012, 116(48): 25493-25503.
陈彩花, 彭海龙, 梁利芳, 等. 燃烧法可控制备Eu 3+-Eu 2+共存Ca12Al14O32F2荧光粉及其荧光性能的研究 [J]. 发光学报, 2016, 37(8): 932-939.
[16] Peng H L, Gao Q S, Meng L L, et al. Sol-gel method and optical properties of Ca12Al14O32F2∶Eu 3+ red phosphors [J]. Journal of Rare Earths, 2015, 33(9): 927-932.
Peng H L, Gao Q S, Meng L L, et al. Sol-gel method and optical properties of Ca12Al14O32F2∶Eu 3+ red phosphors [J]. Journal of Rare Earths, 2015, 33(9): 927-932.
[18] Zhang C M, Yang J, Lin C K, et al. Reduction of Eu 3+ to Eu 2+ in MAl2Si2O8 (M=Ca, Sr, Ba) in air condition [J]. Journal of Solid State Chemistry, 2009, 182(7): 1673-1678.
Zhang C M, Yang J, Lin C K, et al. Reduction of Eu 3+ to Eu 2+ in MAl2Si2O8 (M=Ca, Sr, Ba) in air condition [J]. Journal of Solid State Chemistry, 2009, 182(7): 1673-1678.
陈彩花, 杨国辉, 蒙丽丽, 张丽霞, 梁利芳. 碱土金属离子共掺杂对铕离子在Ca12Al14O32F2中发光性能的影响[J]. 光学学报, 2017, 37(10): 1030001. Caihua Chen, Guohui Yang, Lili Meng, Lixia Zhang, Lifang Liang. Effect of Alkaline Earth Ion Co-Doping on Photoluminescence from Europium Ion in Ca12Al14O32F2[J]. Acta Optica Sinica, 2017, 37(10): 1030001.






