Upconversion luminescence (UCL) materials have been extensively investigated since the mid-1960s and have found different applications in photonics: solar cell, sensors, detection, solid-state lasers, visualizers, etc. In the last decade, the field of rare-earth (RE) doped upconversion (UC) nanoparticles, powders, and phosphors is rapidly progressing from the fundamental understanding of photoluminescence properties to a lot of applications in medicine and biology[13" target="_self" style="display: inline;">–3].
UC properties of RE ions strongly depend on the host. Highly efficient UCL is observed for fluorite-type materials (, Sr, Ba)[4–9" target="_self" style="display: inline;">–9] because they have low phonon energy ( of ) [10] and the tendency to form multiple cluster configurations even when the doping concentration is low[11–14" target="_self" style="display: inline;">–14]. Low phonon energy allows the lifetime of the intermediate levels to be increased. The clustering effect reduces the distance between ions and thereby increases the probability of an energy transfer process between them, which is beneficial for achieving efficient UCL.
-doped phosphors are demonstrated efficient UCL upon excitation of different infrared energy levels of ions. At present, a large number of papers are devoted to the study of UCL of -doped fluoride and oxide phosphors upon excitation by laser radiation at about 980 nm[15–22" target="_self" style="display: inline;">–22]. UCL of powders prepared by combustion synthesis ions was demonstrated upon excitation of the level of ions by Rakov[15]. However, we have not found publications of the mechanisms and absolute quantum yield of UCL of phosphors.
To develop new UC phosphors for the different applications fields, the nature of UC and luminescence efficiency need to be investigated. Thus, our research is focused on a detailed study of the mechanisms of UCL of phosphors upon 972 nm laser diode (LD) excitation. Also, the -concentration dependence of UCL and the absolute photoluminescence quantum yields of were studied.
The (mole fractions of Er3+ ions are 1.6%, 3.4%, 6.0%, 8.8%, 14.2%, 18.3%, and 21.3%) phosphors were synthesized by using a co-precipitation with the aqueous nitrate solution technique[2325" target="_self" style="display: inline;">–25]. The initial reagents for the synthesis of fluoride powders were strontium nitrate (99.99% for metallic impurities), erbium nitrate five hydrate (99.99% for metallic impurities) produced by LANHIT (Moscow, Russia), ammonium fluoride, and double distilled water. An erbium and strontium nitrate aqueous solution of 0.08 M concentration was added dropwise to a 7% excess of 0.16 M aqueous ammonium fluoride under intense stirring. After precipitation of solid solution the matrix solution was decanted. The obtained powders were dried in air at 45°C (5 h) and annealed in platinum crucibles in air at 600°C (1 h).
The luminescence of the ions excited by an LD at 972 nm was recorded using a Horiba FHR1000 spectrometer. The focused excitation beam diameter on the samples was 712 μm. The incident excitation power was 100 and 250 mW.
The luminescence rise and decay were recorded from , , , , , , , and levels of . For excitation of the level, we used a Ti:sapphire laser model LX329 (Solar LS) at a wavelength of 972 nm. The duration of the exciting pulse was 20 ns. The excitation pulse repetition frequency was 10 Hz. The rise and decay of luminescence were examined by a Tektronix TDS 2022C digital oscilloscope (200 MHz).
The integrating sphere method was used to measure the absolute photoluminescence quantum yield[26,27]. The system consists of the OL IS-670-LED integrating sphere, an OL-770 UV/VIS (Gooch & Housego) spectroradiometer, and a monochromator-spectrograph M833 (Solar LS). The incident excitation power was measured using a UP19K-110F-H9-D0 (Standa) power meter. All measurements were performed at room temperature.
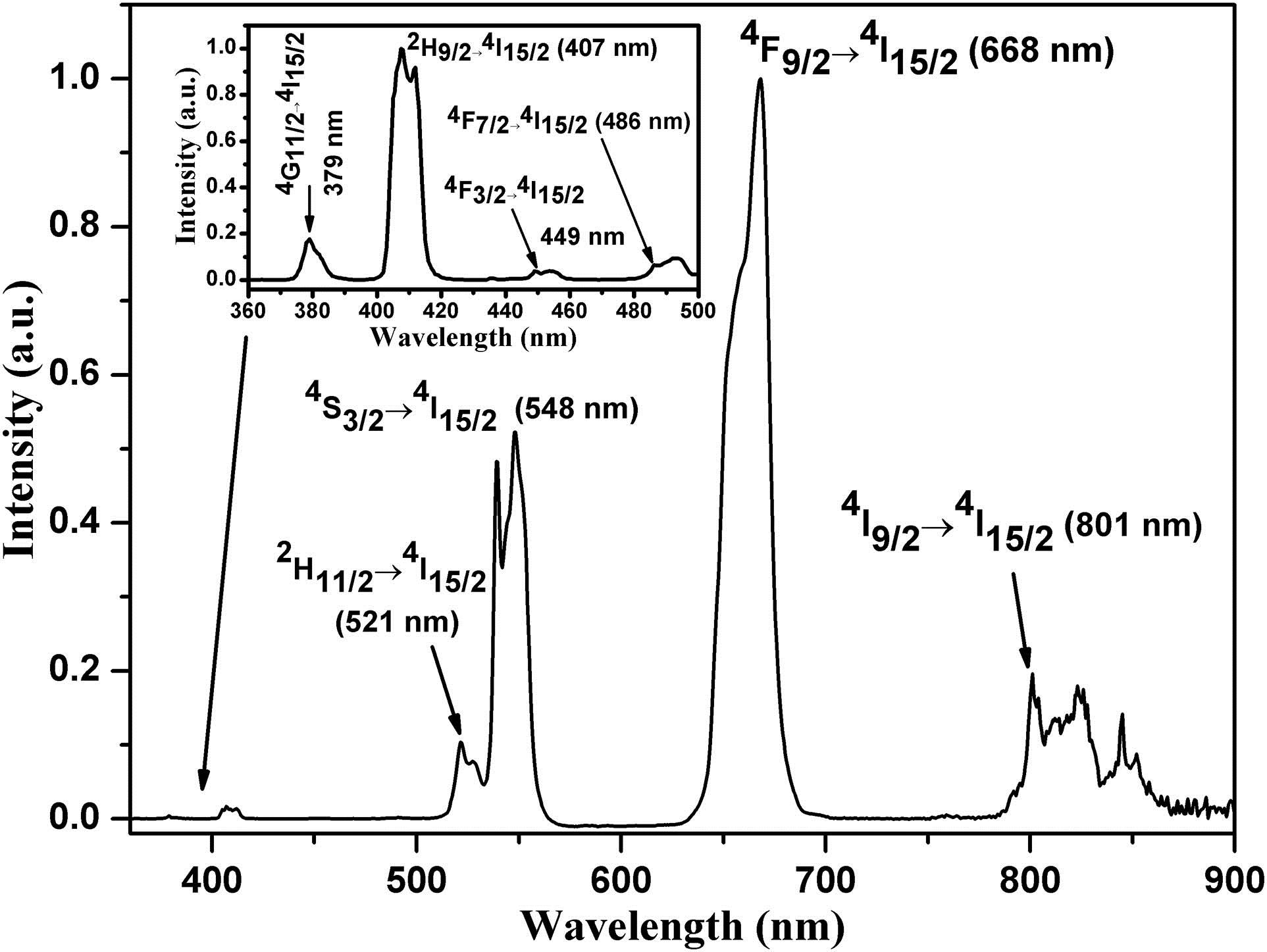
Upon excitation of the level, the visible and near-infrared UCL spectra of ions in phosphors at 300 K corresponding to , , , , , , , and transitions were recorded (Fig. 1). The most intense luminescence was observed in the green and red spectral ranges. The same UCL spectra were observed for all samples. The absorption transition, luminescence transitions, and possible UC mechanisms of ions in phosphors are shown in Fig. 2.
Fig. 1. UCL spectra of (14.2 mol.%) in the visible and near-infrared spectral ranges.
下载图片 查看所有图片
Fig. 2. Absorption transition, luminescence transitions, and UC mechanisms of ions in .
下载图片 查看所有图片
Next, two experimental methods to determine mechanisms of UCL in phosphors were applied. First, excited-state dynamics of phosphors were investigated. Second, we studied the excitation power density (P) dependence of UCL.
From literature it is known that excited-state absorption (ESA), energy transfer UC (ETU), and cooperative processes (CPs) are dominated mechanisms of UCL in -doped materials upon excitation of the level[15–19" target="_self" style="display: inline;">–19,21,22,28,29]. We recorded the rise and decay luminescence of ions from , , , , , , and levels upon excitation of the level. The ESA process leads to an immediate rise of luminescence within the experimental time resolution and a subsequent fast decay corresponding to the relaxation time of the energy level. In contrast, luminescence originating from ETU and CP has a rise part after pulsed excitation. Also, ETU and CP persist after pulsed excitation much longer than the lifetime of the energy level. The nature of the ETU and CP processes is ion–ion interaction of RE ions. Both ETU and CP can simultaneously be responsible for the UCL in a material. But commonly CPs are less effective than ETU ones by 4–5 orders of magnitude[30].
Figure 3 presents the rise and decay of the luminescence of ions from [Fig. 3(a)], [Fig. 3(b)], and [Fig. 3(c)] levels. For all samples, the luminescence from the , , and levels exhibits a slow rise and slow decay after the excitation pulse (20 ns), indicating that ETU processes contribute to populating these levels. The decay time of the luminescence from the , , and levels increases with increasing concentration of ions from 1.6% to 6%. These time dependences are explained by increasing the efficiency of the ETU1 () and ETU2 () processes. Upon further increasing of the concentration of ions, the decay time begins to decrease. Su et al.[28] showed that the lifetime of the intermediate and levels of the ions in the crystals begins to decrease with increasing concentration of ions (approximately from 4 mol.%). This explains the reduced decay time of blue and green luminescence for heavily-doped phosphors.
Fig. 3. Luminescence rise and decay from (a) , (b) , (c) , (d) , (e) , , levels of ions in .
下载图片 查看所有图片
The rise and decay of luminescence from the level were detected [Fig. 3(d)]. The luminescence from the level exhibits a slow rise and slow decay after the excitation pulse. This phenomenon is direct evidence of populating of the level by the ETU process. The dependence of the decay of UCL from the level on the concentration of ions is complex. In our opinion, this is caused by a competition of radiative relaxation () with the populating (ETU3) [, CR1 ()] and depletion (ETU2) processes.
Figure 3(e) presents the decay luminescence from , , and levels for . An almost immediate rise is observed in the time dependence of the UCL of ions from these levels. These experimental results show that the dominant mechanisms in the populating of the and levels are the and , respectively. Energy gaps between and levels as well as and levels correspond to the energy of the incident photon at a wavelength of 972 nm. The fast rise of the UCL from the level is explained by a strong influence of the cross-relaxation (CR1) and multi-phonon relaxation (MPR) on the depletion of this level. The near-infrared UCL from the (801 nm) level appears to correspond to the CR2 between the two levels and ().
To identify the mechanisms responsible for UCL of ions in the upon excitation of the level, we also studied the excitation power density P-dependent UCL at the (548 nm) and (668 nm) transitions of the ions (Fig. 4). It is well known[31] that the UCL intensity depends on the excitation power density P as , where n is the number of absorbed photons needed for populating the upper energy level of the transition. Pollnau et al.[31] investigated in detail the influence of the types of UC mechanisms on the slopes.
Fig. 4. P-dependent UCL at the (a) and (b) transitions of ions. The diagram is in a double logarithmic scale.
下载图片 查看所有图片
Figure 4 shows the log-log dependence of the UCL intensity on the LD excitation power density for the and samples. The slopes of the [Fig. 4(a)] and [Fig. 4(b)] transitions for are calculated to be 2.72 and 2.6, respectively. These results indicate that the and transitions were mainly attributed to three-photon absorption processes at a low concentration of ions (ETU1 or ETU2, ESA1 or ESA2). For (8.8%) the slopes of green and red luminescence are 1.86 and 2.05, respectively. This means that two-photon absorption processes [CP ()] are the dominant mechanisms of visible UCL for phosphors with high concentrations of ions. As mentioned above, the rare-earth ions in have a pronounced tendency to associate in clusters. At low rare-earth () concentrations (on the order of a few hundredths of a percent), oppositely charged point defects and Fint combine to form dipole pairs[32]. Increasing the rare-earth concentration in the solid solution leads to further defect association and defect clustering[13,33]. The concentration of clusters increases with increasing the RE concentration and the phenomenon of percolation begins from 6%[23]. As a result of this phenomenon, clusters come to inevitable spatial contact with each other. Thus, superclusters are formed, which reach a micron size. Rare-earth elements are concentrated in these superclusters. Apparently, phosphors at a high concentration are characterized by an increase in the clusters concentration. The presence of ion–ion interaction between ions in neighboring clusters in these samples leads to an increase in probability of the cooperative process.
Next, the influence of the concentration on the UCL intensity in the visible spectral range was studied. Figure 5(a) presents the spectral power of the UCL of ions in the visible range upon laser excitation at 972 nm for (mole fractions of Er3+ ions are 1.6%, 3.4%, 6.0%, 8.8%, 14.2%, 18.3%, and 21.3%).
Fig. 5. (a) Spectral power of the UCL of . (b) The CIE chromaticity diagram of . The excitation power density is .
下载图片 查看所有图片
It follows from Fig. 5(a) that the strongest UCL occurs when the concentration of ions is 14.2 mol. %. Upon further increase of the concentration of ions, the spectral power of the UCL begins to decrease. The ratio of red to green luminescence of ions is the same for all samples.
phosphors could be used as infrared quantum counters, temperature sensors, visualizers of infrared laser radiation, phosphors for light-emitting diodes, and others. To develop UC phosphors for the above application fields, the luminescence efficiency, i.e., photoluminescence quantum yield needs to be investigated. The photoluminescence quantum yield is defined as the number of emitted photons per that of photons absorbed by luminescence materials. We have developed a system (see Section 2) for measuring UC photoluminescence quantum yield based on an absolute method[26,27].
The photoluminescence quantum yields of (mole fractions of Er3+ ions are 1.6%, 3.4%, 6.0%, 8.8%, 14.2%, 18.3%, and 21.3%) were measured upon excitation of a 972 nm LD with different power densities. The results of the measurement of are shown in Table 1.
Table 1. Quantum Yields, Chromaticity Coordinates x, y and Color Temperature T of SrF2:Er Phosphors
| (mol.%) | P () | (%) | | | T (K) |
|---|
| 1.6 | 25 | – | 0.3497 | 0.3548 | 4838 | | 63 | – | 0.3463 | 0.3470 | 4936 | | 3.4 | 25 | 0.02 | 0.3498 | 0.3599 | 4858 | | 63 | 0.09 | 0.3515 | 0.3643 | 4814 | | 6.0 | 25 | 0.10 | 0.3507 | 0.3722 | 4892 | | 63 | 0.16 | 0.3586 | 0.3888 | 4692 | | 8.8 | 25 | 0.14 | 0.3527 | 0.4207 | 4961 | | 63 | 0.17 | 0.3639 | 0.4268 | 4695 | | 14.2 | 25 | 0.17 | 0.3602 | 0.4795 | 4922 | | 63 | 0.28 | 0.3665 | 0.4753 | 4782 | | 18.3 | 25 | 0.06 | 0.3546 | 0.4118 | 4887 | | 63 | 0.09 | 0.3627 | 0.3924 | 4589 | | 21.3 | 25 | 0.03 | 0.3461 | 0.3765 | 5041 | | 63 | 0.04 | 0.3533 | 0.3712 | 4783 |
|
查看所有表
The absolute quantum yield of phosphors increases with an increasing concentration of ions up to 14.2%. The maximum quantum yield was achieved at 0.28% for (14.2 mol.%) with an incident laser power density of .
The dependences of the quantum yield and intensity of the UCL on the concentration of ions can be explained by UC mechanisms. Important quenching pathways of UCL of are radiative transitions , and of ions. For example, efficient mid-infrared laser oscillations of crystals upon excitation of the level were demonstrated at room temperature[28,29]. The results of investigation of the excited-state dynamics and P-dependent UCL of the phosphors show that the probability of CP increases with increasing concentration of ions. CP depopulates the level and populates the visible levels of the ions. Thus, with an increasing concentration up to 14.2%, CP leads to a decrease in the mid-infrared quenching pathway of the UCL of and thereby increases the photoluminescence quantum yield. Reducing the quantum yield and intensity of the UCL of upon further increasing the concentration of ions is explained by concentration quenching.
Increasing the power density leads to enhancing the UC quantum yield of because the probability of the UC processes also increases.
The chromaticity of the phosphors was calculated by use of the Commission International de l’Eclairage (CIE) chromaticity coordinates (, ) and the results are presented in Fig. 5(b) and Table 1. The color temperatures for with 1.6%, 3.4%, 6.0%, 8.8%, 14.2%, 18.3%, and 21.3% concentrations of ions were 4936, 4814, 4692, 4695, 4782, 4589, and 4783 K, respectively.
In summary, the mechanisms of UCL of phosphors corresponding to the , , , , , , and transitions upon excitation of the level of ions were investigated for the first time. ETU processes are responsible for populating the , , , and levels. CP is the dominant mechanism of UCL from the and levels for high concentrations of ions. The UCL from and is explained by ESA. Cross-relaxation processes play a significant role in populating the and levels. For quantifying material performance the -concentration dependence of UCL and absolute quantum yields of phosphors were studied. The most intensive visible luminescence was obtained for (14.2 mol.%) with a 0.28% maximum quantum yield. The present results indicate that prepared by using a co-precipitation from the aqueous nitrate solution is a promising UC phosphor.
A. A. Lyapin, S. V. Gushchin, A. S. Ermakov, S. V. Kuznetsov, P. A. Ryabochkina, V. Yu. Proydakova, V. V. Voronov, P. P. Fedorov, M. V. Chernov. Mechanisms and absolute quantum yield of upconversion luminescence of fluoride phosphors[J]. Chinese Optics Letters, 2018, 16(9): 091901.
 Download: 537次
Download: 537次