Highly luminescent and stable lead-free cesium copper halide perovskite powders for UV-pumped phosphor-converted light-emitting diodes  Download: 988次
Download: 988次
1. INTRODUCTION
Metal-halide perovskite materials have attracted significant attention over the past decades owing to their advantages of high absorption coefficient, large carrier diffusion lengths, and superior photoelectric properties [1–
The ball milling approach based on mechanochemistry, a kind of green and reemerging efficient synthetic method, was identified by the International Union of Pure and Applied Chemistry (IUPAC) as one of 10 world-changing technologies [49]. The process can promote physical and chemical reactions between solids quickly and quantitatively with no added solvent, consistent with sustainable development. Moreover, the ball milling method offers tremendous advantages compared to traditional solution-based methods by avoiding the solubility limitation for poorly soluble or insoluble reagents and achieving high yield in a relatively short time by controlling materials [50]. Recently, it has been adopted to fabricate lead halide perovskite materials [51,52]. However, there are no reports to our knowledge about using the ball milling approach to fabricate cesium copper halide perovskites. In this work, we first extended the ball milling method to prepare highly luminescent and stable and perovskite powders without solvent. The as-fabricated all-inorganic copper-based perovskites exhibit self-trapped excitons (STE) emission features including broad photoluminescent (PL) emission, large Stokes shift, long PL lifetime, and high PL quantum yield (QY) reaching 60%. The and perovskites with good thermal stability and photostability were employed as phosphors for UV-pumped phosphor-converted (pc)-LEDs.
2. EXPERIMENT
2.1 A. Materials and Synthesis
The materials used were cesium iodide (CsI, 99.9% metal basis, Aladdin), cuprous iodide (CuI, 99.9% metal basis, Aladdin), cesium chloride (CsCl, 99.5%, Macklin), and cuprous (I) chloride (CuCl, 99.5%, Macklin). All chemicals were used as received without further purifications. powders were fabricated by the dry ball milling method at room temperature. In typical synthesis of powder, 3 mmol CsI (0.779 g) and 2 mmol CuI (0.38 g) were first homogeneously mixed in a mortar; the mixture was then transferred into a grinding tank (steel bowl with steel ball, 10 mL). The sealed tank was installed in the vibratory ball mill, and the gray-white powder was obtained by grinding at 1000 r/min for half an hour. For the synthesis of powder, CsI and CuI were simply replaced by CsCl and CuCl in the same process.
2.2 B. Fabrication of UV-Pumped pc-LEDs
0.05 g of powder and 0.05 g of powder were mixed with a thermal-curable silicone resin OE-6551A (0.1 g) under vigorous stirring. The hardener OE-6551B (0.2 g) was added to form a fluorescent paste, and then the paste was deposited on a commercial GaN-based UV-LED chip (310 nm, EPILED Co., Ltd).
2.3 C. Characterization
The morphologies and elemental analysis of and were collected by scanning electron microscope (SEM), energy dispersive X-ray (EDX, FEI Quanta FEG 250 ESEM), and transmission electron microscope (TEM) (JEOL, JEM-2010F, 200 kV) equipped with an X-ray spectrometer detector. X-ray diffraction (XRD) patterns of and powders were recorded on an X-ray diffractometer (Bruker AXS D8) using X-ray radiation (). Thermogravimetric analysis (TGA) results of the powder were obtained using a PerkinElmer Diamond TG/DTA6300, conducted at a heating rate of to 1500°C in flow with an alumina crucible. X-ray photoelectron spectroscopy (XPS) measurements were performed on a ULVAC-PHI instrument (PHIQUAN-TERA-II SXM) with Al as the X-ray source at 70 W. The UV-Vis diffuse reflectance spectra of the powdered samples were taken on a PerkinElmer Lambda 35 double-beam spectrometer. PL and PL excitation (PLE) spectra were recorded on the Horiba PTI QuantaMaster 400. The absolute PL QY’s time-resolved PL decay curves were measured on an FLSP920 spectrofluorimeter (Edinburgh Instruments, TCSPC system) equipped with an integrating sphere.
2.4 D. Computational Methods
First-principle calculations of were carried out using the Vienna Ab initio Simulation Package (VASP) code. To guarantee convergence, the projected augmented plane wave basis set was defined by a cutoff of 300 eV. The mesh samplings in the Brillouin zone (BZ) were . Experimental lattice parameters of were used, and the atomic positions were fully relaxed until the residual forces were 0.05 eV/Å. Electronic band structures, density of states (DOS), and exciton properties were calculated using the hybrid PBE0 function.
3. RESULTS AND DISCUSSION
The schematic procedure of a typical fabrication of powder by using a planetary ball mill is illustrated in Fig.
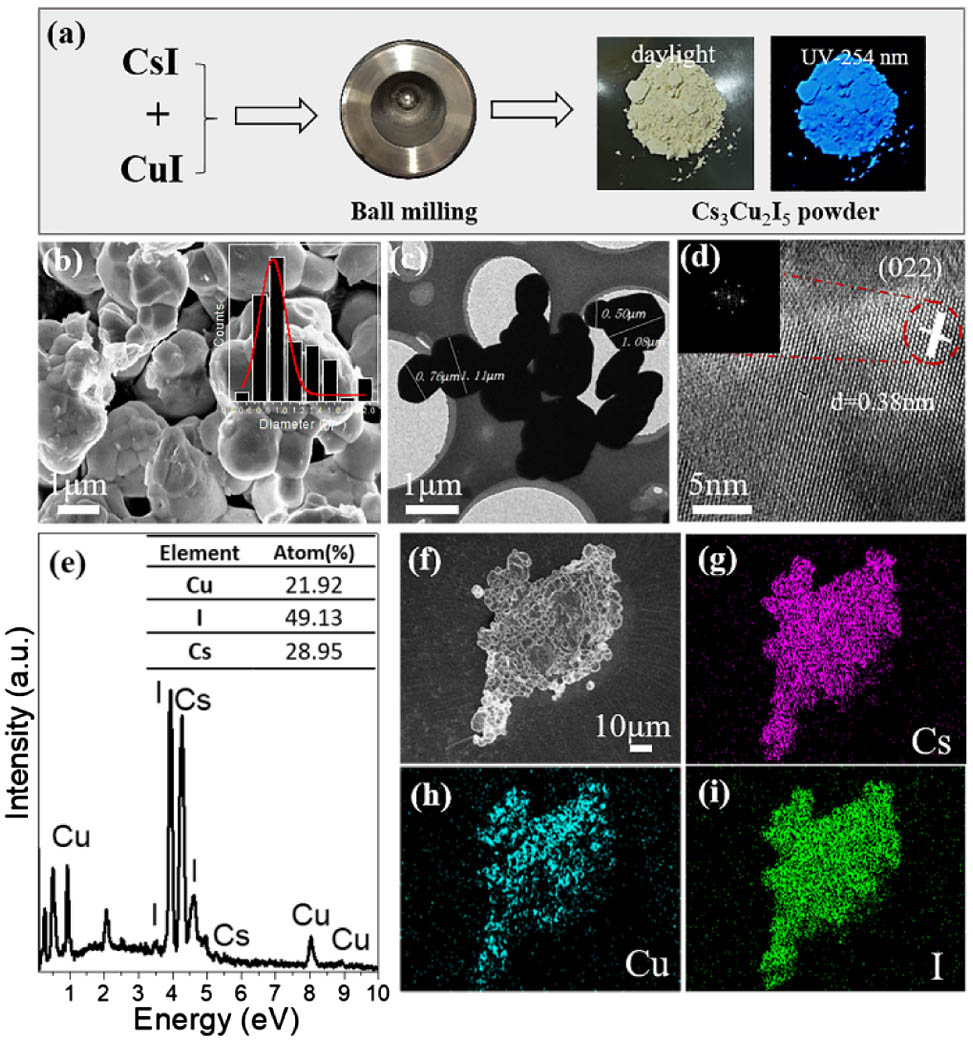
Fig. 1. (a) Schematic illustration of synthetic process of
We performed XRD measurement to verify the phase structure of the as-synthesized ; as shown in Fig.

Fig. 2. (a) XRD pattern of obtained
The absolute PL QY of was measured up to 60%, indicating that our sample has a strong emission feature. Figure

Fig. 3. (a) PL spectra of
The thermal stability and photostability of perovskite materials are critical for their long-term application in lightings and displays. To evaluate the natural stability of powder, the evolution of the PL spectra of powder after thermal treatment under protection at different temperatures (100°C, 200°C, 300°C) was tested for half an hour. It can be seen clearly from Fig.

Fig. 4. (a) Integrated PL intensity as a function of temperatures from 25°C to 300°C. Variation of PL intensity of
Notably, green emissive perovskite was achieved for the first time to our knowledge. The SEM image is shown in Fig.

Fig. 5. (a) SEM image of
The UV-Vis absorption and PL spectra of powder are depicted in Fig.

Fig. 6. (a) Normalized UV-Vis absorption (purple dash line) and PL (green solid line) spectra of the as-obtained
In order to illustrate the potential lighting application of the obtained perovskite powders, we fabricated a UV-pumped pc-LED device by using blue emissive and green emissive as phosphors. It is the first time to our knowledge that pc-LED based on all copper-based perovskites without other phosphors has been prepared. Figure

Fig. 7. (a) Photograph of the as-fabricated pc-LED based on dual phosphors of blue emissive
4. CONCLUSIONS
In summary, we have developed a simple and energy-saving route to synthesize stable lead-free perovskites in a dry ball milling process. The obtained blue emissive powder exhibits a high PL QY of 60% with a long lifetime of 1.13 μs and a huge Stokes shift of 137 nm. The luminescence mechanism of could be explained by self-trapped excitons that originate from Jahn–Teller distortion of the Cu tetrahedral site. The green emissive perovskite with PL QY of 53% was successfully fabricated by using the same process for the first time, with the PL peak at 510 nm. We finally realized a UV-pumped LED device by using blue emissive and green emissive as phosphors.
[33] L. Zhou, J. F. Liao, Z. G. Huang, J. H. Wei, X. D. Wang, H. Y. Chen, D. B. Kuang. Intrinsic self-trapped emission in 0D lead-free (C4H14N2)2In2Br10 single crystal. Angew. Chem. (Int. Ed.), 2019, 58: 2-8.
Article Outline
Lingling Xie, Bingkun Chen, Fa Zhang, Ziheng Zhao, Xinxin Wang, Lijie Shi, Yue Liu, Lingling Huang, Ruibin Liu, Bingsuo Zou, Yongtian Wang. Highly luminescent and stable lead-free cesium copper halide perovskite powders for UV-pumped phosphor-converted light-emitting diodes[J]. Photonics Research, 2020, 8(6): 06000768.





