The photoluminescence (PL) spectrum of ions is known as being composed of several narrow lines, as is usual for many other trivalent rare-earth (RE) ions due to the well-shielded 4f-4f shell transitions[13" target="_self" style="display: inline;">–3]. The most intense emission of is at about 542 nm, which corresponds to a transition from the lowest excited state () to the ground state () above the lowest ground state level [1,4]. On the other hand, the absorption of in the deep UV region is relatively weak compared with the near-UV lines[5]. In fact, investigations on luminescent materials to be coupled with the deep UV LED chip have great potential because of the rapid development of group III nitride-based UV diodes[6]. To achieve an enhanced luminescence action with for deep UV excitation, it might be of use in an experimental effort by introducing so-called sensitizer ions, which have a strong absorption in this region[5]. There has been quite a lot of work in this respect about terbium sensitization by doping other REs in inorganic solids. For example, an enhanced energy transfer (ET) mechanism from cerium (), gadolinium (), or dysprosium to terbium () has been established in a variety of phosphors or glasses[5,7,8]. Meanwhile, sensitization of luminescence has also been successfully tried by co-doping ions other than REs in different hosts, such as recently reported work on in borosilicate glass[9].
In this Letter, we wish to report the experimental observations where luminescence for the deep UV excitation is sensitized by co-doping non-RE ions in phosphate glasses. As the host in the present work, the phosphate glass possesses the advantage of higher solubility for ions, for example, up to 10 mol.% doping without causing concentration quenching of emission[5], and the wide transmission range from the deep UV to the near-IR region. As a sensitizer, exhibits a wide and strong absorption band in the deep UV region due to transitions[10]. Since the energy level of is energetically close to the higher excited state of (), it is especially expected that the resonance of the excitation with the deep UV light source could be greatly enhanced via ions co-doping. Besides possibly playing a sensitization role for luminescence under the deep UV excitation, the ion also acts as a co-activator by absorbing UV energy to emit in the blue to green light region[10]. Thus, emissions from and ions could be adjusted easily by changing the concentration ratio to obtain the required composite light with the specific CIE chromaticity coordinates for different applications.
The nominal composition of the host glass used for the present work is [10], while dopants () are introduced in two groups with the concentrations (mol. %) given in Table 1. Samples in the first group contain the varied concentration from 0 to 2.0 (G1, G3-G6) while fixing the concentration constant at 0.2, whereas those in the second group keep the same concentration of at 2.0 but increased the from 0 to 0.8 (G2, G6-G9). The sample G10 was additionally prepared in order to demonstrate the effect of the varied concentration ratio on the CIE chromaticity coordinates of emission.
Table 1. Extra Tb, SnO Doping Concentrations (mol.%)
| Samples | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | G9 | G10 |
|---|
| 0.2 | 0.0 | 0.2 | 0.2 | 0.2 | 0.2 | 0.4 | 0.6 | 0.8 | 0.8 | | 0.0 | 2.0 | 2.0 | 1.5 | 1.0 | 0.5 | 2.0 | 2.0 | 2.0 | 1.0 |
|
查看所有表
Glass samples were prepared using the melt-quenching method. The starting materials are chemical purity compounds including , , and SnO, as well as elemental Tb. The as-prepared glass batches according to Table 1 were mixed thoroughly and uniformly, and then poured into crucibles for melting in an electric furnace at 1250°C for 2–3 h. The melts were quenched in air, and all as-prepared glasses were then annealed at 450°C for 2–3 h and finally cut into rectangular shapes with 2 mm thickness and polished to mirror smoothness and ready for optical measurements.
All glass samples are transparent in the UV and visible regions as characterized by their absorption spectra (omitted herein). PL emission and excitation spectra were collected by a high-resolution spectrofluorometer (Fluorolog-3, Horiba Jobin Yvon Inc., Edison, NJ) using a 450 W Xe lamp as the excitation source. The PL decay lifetime was measured by FLSP920 (Edinburgh Instruments, Livingston, UK) using an nF900 μs pulsed Xe lamp as the source with a pulse width of 2–3 μs. All measurements were carried out at room temperature.
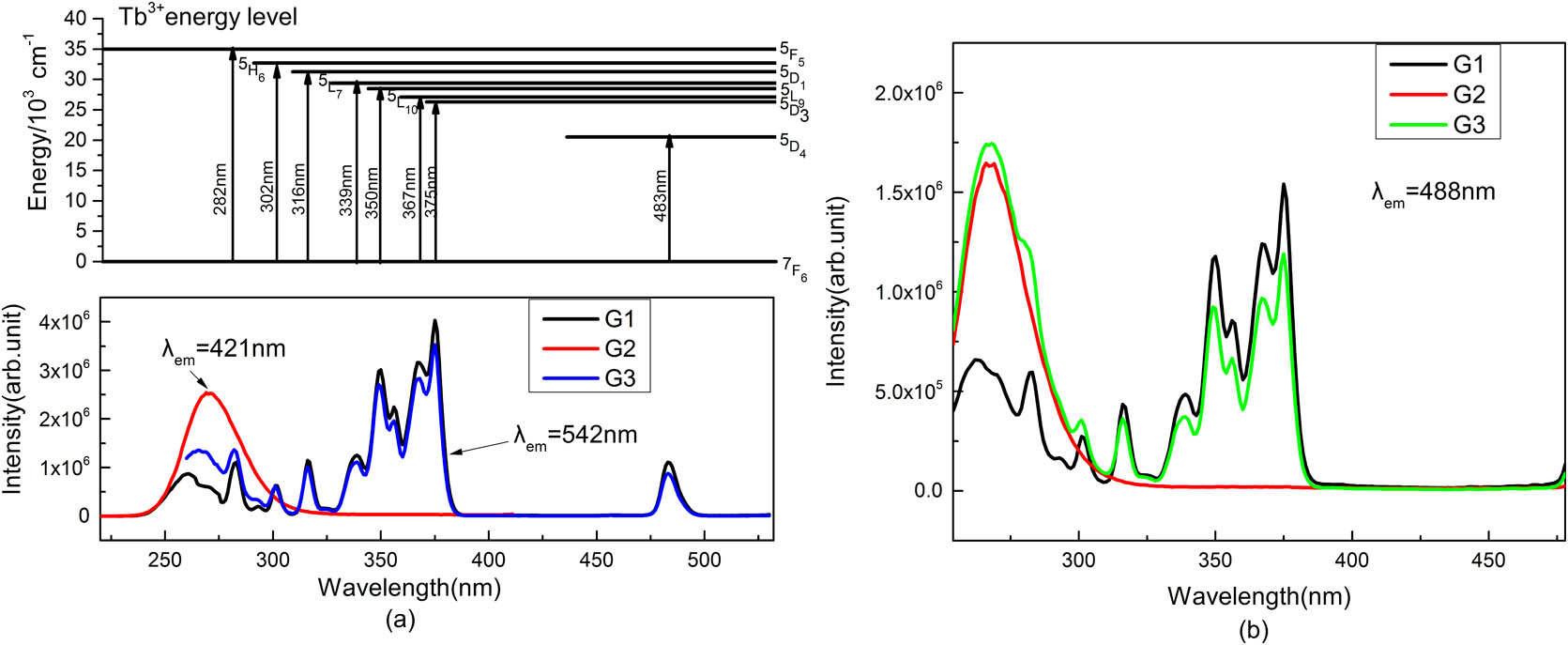
Figure 1 presents PL excitation (PLE) spectra of , singly doped (G1, G2) and co-doped (G3) samples for emissions of and/or ions. As shown in Fig. 1(a), several narrow excitation lines are observed in G1 for the 542 nm emission, with corresponding energy levels given in the figure for clear illustrations of the electronic transition characters of ions[1,4,5,11]. For the 421 nm emission in G2, a broad and strong excitation band appears in the deep UV region peaking at around 270 nm due to the transition of , which agrees well with the results of the previous work[10]. For the case of G3, by monitoring the emission at 542 nm where has a small but sizable emission, there remain the narrow excitation lines of . However, the intensity ratio of the deep UV to the near-UV excitation increases obviously due to the superimposed excitation of and in this region. PLE spectra are monitored at 488 nm where and emissions are more largely overlapped. It is interesting to note from Fig. 1(b) that, relative to G1, G3 shows a tremendously enhanced deep UV excitation peaking at 270 and 282 nm, respectively. In particular, the 270 nm excitation band well resembles that for the emission of G2 in shape, implying convincingly the role of as a sensitizer for enhancing the deep UV excitation of in the present phosphate glass.
Fig. 1. PLE spectra of G1-G3 monitored, respectively, at (a) 421/542 nm and at (b) 488 nm.
下载图片 查看所有图片
For characterizing PL spectra of samples G1-G3, we choose 375 and 282 nm as the near and deep UV excitation sources, respectively, where 282 nm (instead of 270 nm) was used for the purpose of avoiding frequency doubling in the main emission region (542 nm). It follows from Fig. 2(a) that with G2 is not excited, while G1 and G3 show the same PL spectra characteristic for electronic transition behaviors[11,12]. This demonstrates that co-doping exerts no negative effect on emissions for near-UV excitation. On the other hand, with [Fig. 2(b)], emission bands in G3 are all superimposed onto the emission band. To identify variations of the emission intensity between G3 and G1, GAUSSIAN fitting was made on the PL spectrum of G3, and the fitted curve after subtracting the emission is shown in the inset of Fig. 2(b) where the PL spectrum of G1 is presented for comparison. It is clear that, relative to G1, the emission intensity of G3 increases in general, especially at 542 nm, evidencing the role of as a sensitizer for emission under deep UV excitation. To describe the possible ET process from to , the simplified diagrams of energy levels for and are jointly presented in Fig. 2(c). It is seen that there most likely exists an ET channel to from due to the quasi-resonance in energy of the level with the center: level. That is, an excitation of ions from the ground state to the excited state produces the emission of ions at 421 nm. It then results in population of excited state from the ground state due to cross relaxation, yielding the enhanced emission at 602 nm.
Fig. 2. (a, b) PL spectra and (c) simplified energy level diagrams of and .
下载图片 查看所有图片
It is also observed from Fig. 2(b) that the broad emission appearing in G3 is almost the same as that in G2, suggesting that the depression of the entire emission spectrum of the sensitizer did not occur. It thus means that the radiative ET from to is involved under the deep UV excitation of the present co-doped phosphate glass[5]. To confirm this conclusion, the decay rate of the emission in G3 was measured and the result is compared with that of G2 in Fig. 3(a). It is seen from Fig. 3 that the PL decay curves of both G2 and G3 for the emission are single exponentially fitted well, indicating that also acts as an independent activator for blue emission. The emission lifetime () is calculated from the fitting equation , where is the luminescence intensity, is the constant, and is the time, and the result does not show an obvious difference in the value between G3 (6.32 μs) and G2 (6.34 μs), supporting the suggested radiative transfer mechanism.
To verify the origin of the sensitized green emission in G3, the decay curves for the 542 nm emission were measured on G1 and G3 as well, and the results are compared in Fig. 3(b). Similar to Fig. 3(a), the green emission decay curves of G1 and G3 follow a single exponential decay function, with the emission lifetime calculated, respectively, as 2.88 ms (G1) and 2.94 ms (G3). This is typical for the forbidden f-f transitions of , which is clearly reminiscent of the green emission in G3 originating independently from , although sensitized by . The PL lifetimes of all samples are summarized in Table 2, where values of the green (542 nm) and blue (421 nm) emissions in singly doped (G1-G2) and co-doped (G3-G9) samples are all reasonably fluctuated in the millisecond (ms) and microsecond (μs) time regimes, respectively, identical to the foregoing discussions.
Table 2. Equivalent Decay Times of Sn2+ (421 nm) and Tb3+ (542 nm) Emissions for Samples G1-G9
| | PL Decay Rate Measurements |
|---|
| Sample Code | (nm) | (nm) | |
|---|
| G1 | 375 | 542 | 2.89 ms | | 282 | 2.88 ms | | G2 | 270 | 421 | 6.34 μs | | G3 | 375 | 542 | 2.99 ms | | 2823 | 2.94 ms | | 270 | 421 | 6.32 μs | | G4 | 282 | 542 | 3.05 ms | | G5 | 2.96 ms | | G6 | 3.01 ms | | G7 | 270 | 421 | 6.32 μs | | G8 | 6.39 μs | | G9 | 6.33 μs |
|
查看所有表
Figure 4(a) presents additional PL spectra of samples G4-G6 for the 282 nm excitation where the emission at 542 nm is enhanced regularly with increased doping. It demonstrates consistently the close tie of the emission with the presence of . Meanwhile, the intensity ratio of green to blue emissions increases gradually with the decreased , or, on the other hand, with the increased [Fig. 4(b)]. As examples, PL spectra of several typical samples (G1-G3, G9-G10) are characterized by the CIE chromaticity diagram in Fig. 4(c) together with the luminescent photos of the samples. CIE chromaticity coordinates of emissions for all samples are given in Table 3. It is seen that PL spectra can be tuned from blue to green by adjusting the concentration ratio, thus achieving the tunable composite emission with different chromaticity coordinates of emission for the deep UV excitation.
Fig. 4. PL spectra of (a) G3-G6 and (b) G3, G7-G9, and (c) a CIE chromaticity diagram for G1-G3 and G9-G10 together with photos of luminescent samples.
下载图片 查看所有图片
Table 3. CIE Chromaticity Coordinates of Emissions for Samples G1-G10
| | CIE Chromaticity Coordinates |
|---|
| Sample Codes | | Coordinates |
|---|
| G1 | 375, 282 | (0.261,0.443), (0.283, 0.395) | | G2 | 270, 282 | (0.180,0.173), (0.172, 0.144) | | G3 | 282 | (0.183, 0.182) | | G4 | 282 | (0.187, 0.191) | | G5 | 282 | (0.191, 0.202) | | G6 | 282 | (0.196, 0.217) | | G7 | 282 | (0.192, 0.215) | | G8 | 282 | (0.200, 0.241) | | G9 | 282 | (0.207, 0.265) | | G10 | 282 | (0.230, 0.339) |
|
查看所有表
Benefited energy harvest of ions under deep UV excitation is realized by co-doping ions in doped phosphate glass. PL, PLE, and decay lifetime data consistently present evidence of terbium sensitization in the presence of ions via the radiative ET from to . The mechanism involved is associated with the strong deep UV absorption of , which greatly enhances the resonance of the excitation with the deep UV light source. The tunable luminescence from blue to green is achieved in the co-doped phosphate glass by adjusting the concentration ratio. Our work indicates potential applications of co-doped phosphate glasses as converting phosphors pumped by deep and near-UV LED chips.
Lei Li, Yang Wang, Duojin Wang, Jian Qi, Fanshu Xia, Huidan Zeng, Guorong Chen. Sensitization of Sn2+ on Tb3+ luminescence for deep UV excitation in phosphate glasses[J]. Chinese Optics Letters, 2016, 14(7): 071601.