自适应光学在超分辨荧光显微镜中的应用【增强内容出版】
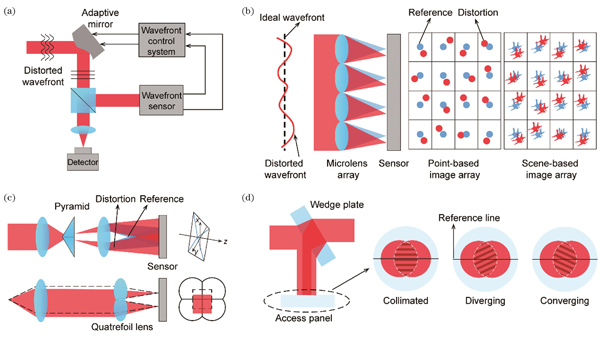
Because of the wave characteristics of light, conventional fluorescence microscopy is typically restricted by the diffraction limit, which is approximately 200 nm laterally and 500 nm axially. Super-resolution microscopy has overcome this barrier and improved the imaging resolution to a few nanometers, which enables the observation of biological structures at a nanoscale and revolutionizes the development of life sciences. Super-resolution microscopy can be classified into three types. The first type is scanning imaging based on point spread function (PSF) decoration, whose representative technique is stimulated emission depletion (STED). The second is wide-field imaging based on spectrum spread, whose representative technique is structured illumination microscopy (SIM). The third is single-molecule localization microscopy (SMLM), also known as photoactivated localization microscopy (PALM) and stochastic optical reconstruction microscopy (STORM). In super-resolution fluorescence microscopy, both instrumentation and sample-induced aberrations decrease the spatial resolution and degrade the imaging quality. Therefore, the adaptive optics (AO) technique is applied, which detects aberrations using direct or indirect methods, and performs compensation through wavefront correction elements to capture high-quality super-resolution images (Fig.1). This review introduces the origin and working principle of AO, summarizes its application in super-resolution fluorescence microscopy, and highlights its future development prospects.
In STED microscopy [see Fig.2(a)], aberrations in the excitation, depletion, and emission paths influence the image quality simultaneously, particularly in the depletion path, which need to be corrected with AO. In 2012, Gould et al. proposed the first implementation of AO in STED microscopy, which used modal sensing with the sharpness metric to examine the aberrations, and performed corrections with two spatial light modulators (SLMs) in both excitation and depletion paths [see Fig.2(b)]. They imaged fluorescence beads at a depth of 25 μm above the retina sections of a zebrafish, with an axial resolution of 250 nm. In 2014, Lenz et al. proposed an off-axis holography configuration that used one SLM to correct instrumentation and sample-induced aberrations [see Fig.2(d)]. They achieved a lateral resolution of 120 nm and an axial resolution of 173 nm when imaging tubulin at depths of 8?10 μm. In 2016, Patton et al. proposed an implementation that incorporates two AO elements to enable aberration correction in all three beam paths [see Fig.2(e)]. They used modal sensing with the Fourier ring correlation (FRC) metric and resolved glutamatergic vesicles in neural boutons in intact brains of Drosophila melanogaster at a depth of 10 μm. In 2019, Zdankowski et al. proposed an automated AO solution to correct instrumentation and sample-induced aberrations. They used modal sensing with the brightness metric and achieved super-resolution imaging of a 15 μm mitotic spindle with a resolution of 50 nm×50 nm×100 nm. On this basis, in 2020, Zdankowski et al. combined AO with image denoising algorithm by block-matching and collaborative three-dimensional (3D) filtering (BM3D) to enhance the image quality and super-resolved 3D imaging of axons in differentiated induced pluripotent stem cells growing under an 80 μm thick layer of tissue with lateral and axial resolutions of 204 and 310 nm, respectively [see Fig.3(a)]. In 2020, Antonello et al. proposed using wavelet analysis to quantify resolution loss and established a multivalued image quality metric. They achieved super-resolution imaging of CA1 pyramidal neurons in an organotypic hippocampal slice at a depth of 14 μm. In 2021, Hao et al. combined AO with 4Pi-STED. They used two deformable mirrors (DMs) in the two paths and analyzed the aberrations with modal sensing [see Fig.4]. They achieved sub 50 nm isotropic resolution of structures, such as neuronal synapses and ring canals previously inaccessible in tissues. Other indirect or direct wavefront detection techniques have been also used to measure aberrations in STED microscopy.
In SIM [see Fig.6(a)], aberrations in excitation and emission paths decrease the imaging quality and should be corrected with AO. In 2008, Débarre et al. implemented sensorless AO in SIM. They investigated how the image formation process in this type of microscopy is affected by aberrations and performed aberration correction with modal sensing. In 2015, Thomas et al. combined sensorless AO with SIM and achieved super-resolution imaging of 100 nm fluorescence beads fixed beneath a C. elegans sample with a 140 nm resolution. In 2021, Zheng et al. proposed an AO correction method based on deep learning and utilized the method to correct aberrations with SLM, realizing super-resolution imaging of phalloidin-labeled actin in cultured BHK cells. Recently, direct wavefront sensing has also been used in SIM. In 2019, Turcotte et al. applied AO in SIM in vivo by generating the guide star with two-photon excitation as the input of the Shack-Hartmann wavefront sensor and performing aberration correction with a DM. They imaged the brains of live zebrafish larvae [see Fig.5(a)] and mice and observed the dynamics of dendrites and dendritic spines at nanoscale resolution. Similarly, in 2020, Li et al. used AO in optical-sectioning SIM [see Fig.6(b)] and achieved fast, high-resolution in-vivo imaging of mouse cortical neurons at depths of 21?29 μm [see Fig.5(b)] and zebrafish larval motor neurons at depths of 10?110 μm. In 2021, Lin et al. used direct wavefront sensing in SIM with a configuration that can be switched among wide-field imaging, structured illumination, and confocal illumination [see Fig.6(c)]. They used modal sensing to correct the aberrations of fluorescence beads and then recorded the image arrays in Shack-Hartmann wavefront sensor as a reference. Subsequently, they used confocal illumination to generate the guide star, input it into the Shack-Hartmann wavefront sensor, and reconstructed the wavefront. They decreased the peak-valley values of the wavefront amplitude from 1.5 to 0.1 μm when imaging C.elegans.
In SMLM [see Fig.7(a)], aberrations in the emission path result in distorted PSFs and decreased localization precision, which should be corrected with AO. In 2015, Burke et al. proposed a technique for correcting aberrations using modal sensing with the sharpness metric [see Fig.7(c)]. They achieved a resolution of 78 nm laterally and 136 nm axially for microtubules at a depth of 6 μm. Tehrani et al. optimized aberrations using genetic algorithm [see Fig.7(d)] with the intensity-independent Fourier metric and increased the localization precision by four times at a depth of 50 μm. In 2017, Tehrani et al. proposed a real-time wavefront aberration correction approach based on particle swarm optimization [see Fig.7(e)] and the intensity-independent Fourier metric. They achieved a resolution of 146 nm for the central nervous system of Drosophila melanogaster at a depth of 100 μm. In 2018, Mlodzianoski et al. developed adaptive astigmatism using Nelder-Mead simplex algorithm to correct wavefront distortions with the weighted sharpness metric. They achieved a resolution of 20 nm laterally and 50 nm axially for mitochondria at a depth of 95 μm. In 2021, Siemons et al. proposed robust and effective adaptive optics in localization microscopy (REALM) combined with modal sensing using the weighted sharpness metric [see Fig.7(f)]. They achieved an FRC resolution of 76 nm for microtubules [see Fig.8(a)] and cytoskeletal spectrin of the axon initial segment at a depth of 50 μm. In 2023, Zhang et al. proposed deep-learning-driven adaptive optics (DL-AO) that examined aberrations from detected PSFs using deep neuron network [see Fig.7(g)]. They achieved a resolution of 14?31 nm laterally and 41?81 nm axially for mitochondria [see Fig.8(b)] and dendrites at a depth of 133 μm. In 2023, Park et al. developed closed-loop accumulation of single-scattering (CLASS), which measured complex tissue aberrations from intrinsic reflectance and performed compensation [see Fig.7(h)], and resolved subdiffraction morphologies of cilia and oligodendrocytes in entire zebrafish at a depth of 102 μm, improving the localization precision from 67 nm to 34 nm.
This review summarizes the application of adaptive optics in super-resolution microscopy, including indirect and direct wavefront detection. Indirect wavefront sensing requires no setup modifications, except for inserting the wavefront correction elements, which is economical and practical. However, the low response speed and narrow dynamic range limit its effectiveness for severe distortions. Direct wavefront sensing can provide increased response speed and dynamic range, despite its increasing complexity in instrumentation. The future prospects of AO methods in super-resolution microscopy include increasing the field-of-view, response speed, and imaging depth. We expect that the AO method will be a general option in future super-resolution fluorescence microscopy.
王翔宇, 陈曦, 曹暾, 马冬晗. 自适应光学在超分辨荧光显微镜中的应用[J]. 中国激光, 2024, 51(3): 0307104. Xiangyu Wang, Xi Chen, Tun Cao, Donghan Ma. Application of Adaptive Optics in Super‑Resolution Fluorescence Microscopy[J]. Chinese Journal of Lasers, 2024, 51(3): 0307104.