光声成像技术在早期癌症检测治疗中的潜在应用  下载: 1884次
下载: 1884次
1 引言
世界卫生组织(WHO)于2017年发表的《全球癌症报告2014》中称,全球癌症新增患者和死亡病例在不断增加[1],其中中国包揽近一半新增病例位列全球新增病例第一。2012年全世界新增癌症病例有1400万,死亡人数达到820万。中国新增癌症病例有307万,占全球癌症新增病例的21.8%,死亡人数为220万,占全球死亡总数的26.9%。2015年中国癌症新增病例为429.2万,死亡人数为281.4万。癌症已经成为中国人群最主要的公共健康问题以及死亡的首要原因。2017年CA: A Cancer Journal for Clinicians期刊在线发布的《2017癌症统计》[2]报告指出,男性最易患前列腺癌,其次是肺(支气管)癌和结直肠癌;女性最容易患乳腺癌,其次是肺(支气管)癌和结直肠癌。通过死亡病例来看,肺(支气管)癌是造成男性和女性死亡的罪魁祸首。癌症的治疗关键在于早发现、早诊断以及早治疗。早期癌症多局限在一个器官内,且尺寸小,未浸润或微浸润到周围组织以及血液与淋巴循环系统,手术等治疗后5年存活率达到80%,极大降低了癌症的死亡率。由于当前癌症检测诊断条件限制以及癌症早期无明显体征,发展一种无创、高分辨率、高对比度、深穿透、肿瘤特异性强的检测方法对癌症的诊断、治疗以及预后具有重大意义。
1880年,Bell等[3]在固体中观察到光声效应。20世纪70年代,光声效应被应用于光谱学研究,形成光声光谱学[4-7]。20世纪80年代,光声效应被应用于生物组织成像研究,形成光声层析成像[8-10]。光声成像[11]基于生物组织内部光学吸收差异、以超声为媒介的无损生物成像方法,克服了光学成像技术在成像深度与分辨率上不可兼得的不足,同时有效地补充声学成像技术在对比度和功能性方面的缺陷。利用探测光声波代替光学成像中的光子检测,检测方式避开了光学散射的影响,为深层组织(50~60 mm)影像提供高对比度以及高分辨率,为生物组织的结构形态[12-13]、生理特征[14-15]、病理特征[16-17]、代谢功能[18-19]等成像提供重要检测手段,在生物医学疾病诊断以及在体组织结构和功能成像领域具有广泛的应用前景。
由于癌组织(恶性肿瘤)内不同的化学成分具有不同的光学吸收特性,光声成像利用光致超声的成像机制选择特定的激光波长对癌组织内化学成分进行检测,从而对癌组织进行高对比度的结构成像。癌症的一个重要标志是过量的氧燃烧(即高代谢),高代谢导致组织内血红蛋白浓度、血氧浓度和氧代谢率等发生改变。光声成像可对癌组织内血红蛋白浓度、血氧浓度和氧代谢率[20]等能精细反映组织微小病变的生物参数进行定量分析,从而实现癌组织功能成像[21-23]。早期癌症还未扩散,诊断无需造影剂。光声成像应用在早期癌症诊断上以深穿透深度[24-25] (厘米量级)实现对早期乳腺癌的检测[26-27]、以高分辨率[28] (微米量级)实现对早期肿瘤新生血管(直径小于10 μm)的监测、高成像速度[29-30](亚毫秒)快速对肿瘤边界进行界定[31]、肿瘤特异性强(DNA-RNA、血红蛋白、黑色素等)[32-34]、为肿瘤提供定量光声光谱信息[35-36]等优点实现对肿瘤的形态结构、生理病理特征进行结构与功能成像。肿瘤内源性光声对比剂对生物体不会产生毒副作用,不会对生物体生理过程产生影响且无需监管部门的批准。而外源性的光声对比剂(如有机染料、荧光蛋白、纳米颗粒等)可优化成像系统癌症检测灵敏度,可与靶向分子(如抗体)结合形成特异性分子成像受体从而实现特异性肿瘤检测。因此光声成像技术在软组织肿瘤的早期无损诊断、定位及分期上是行之有效的检测方法,在未来肿瘤的临床应用上具有潜在的应用价值。
2 光声成像原理
2.1 光声成像基本原理
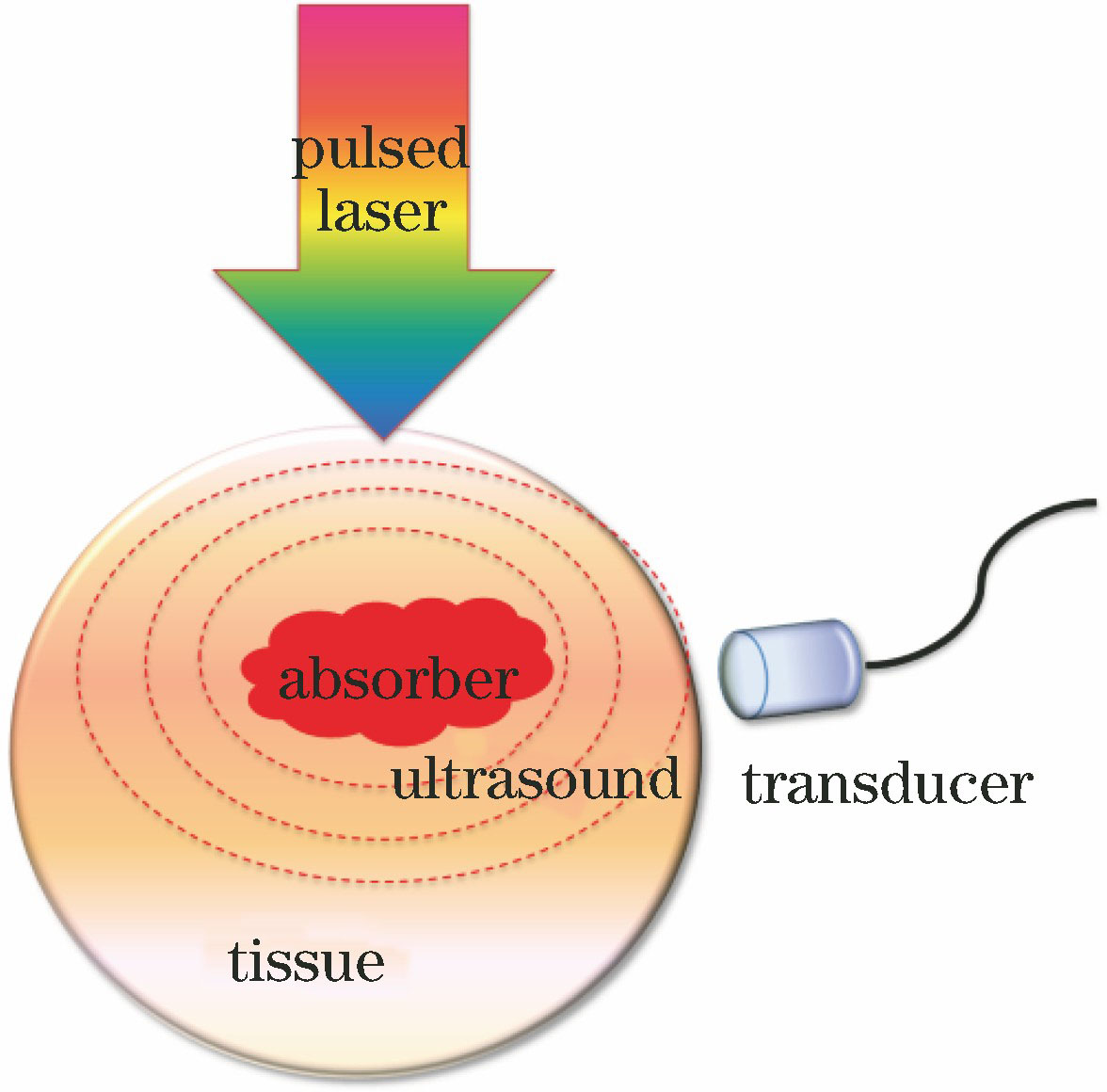
光声成像的原理是光致超声,即光声效应[3]。如
光速远大于声速,因此可将组织内吸收体看成是同时被激发而产生光声压。这种光声压在外传的过程中将被置于不同位置的换能器所接收,从而获得不同的时间延迟信号。再对这种分辨信号进行相应处理便可确定吸收体在组织体内的位置。吸收体的尺寸信息可通过光声双极信号中负极到正极的距离来加以判断[37]。超声阵列检测的吸收体光声信号需要经过特定算法进行重建吸收体信息,算法依赖于超声阵列的扫描方式[38-39]。光声成像的成像机制是热弹性膨胀产生超声波,在满足热限制和压力限制的条件下,各项同性吸收体内光声压
式中:
式中:
当介质内吸收体为球状或柱状时,吸收体吸收分布呈高斯分布,吸收体光声压[43]表示为
式中:
当介质内吸收体为层状结构时,后向探测式光声压[44]表示为
式中:
远场条件下,在
式中:
2.2 光声成像实验装置
光声成像系统依结构形式分为光声计算层析成像(PACT)系统和光声显微成像(PAM)系统。PACT依超声换能器的数目分为单阵元扫描光声成像系统以及多阵元阵列光声成像系统。单阵元扫描光声成像的基本形式是光波经处理后均匀地照射在组织上,然后利用一个单阵元超声探测器通过旋转扫描等方式探测组织内的超声信号,信号经过处理(如放大、取平均等)后,传输到计算机中进行图像重构,成像结果依赖于重建算法。多阵元阵列光声成像系统利用均匀光波照射组织产生光声信号,阵列采集光声信号后进行图像重建。超声阵列以分布结构可分为环形[
![光声成像系统。(a)透射式OR-PAM系统;(b)反射式OR-PAM系统;(c)暗场照明AR-PAM系统;(d)环形超声换能器阵列PACT系统;(e)线性超声换能器阵列PACT系统;(f)半球形超声换能器阵列PACT系统;(g)基于二维法布里-珀罗干涉仪声学传感器的PACT系统;(h)血管内侧视光声导管[46]](/richHtml/lop/2019/56/7/070001/img_2.jpg)
图 2. 光声成像系统。(a)透射式OR-PAM系统;(b)反射式OR-PAM系统;(c)暗场照明AR-PAM系统;(d)环形超声换能器阵列PACT系统;(e)线性超声换能器阵列PACT系统;(f)半球形超声换能器阵列PACT系统;(g)基于二维法布里-珀罗干涉仪声学传感器的PACT系统;(h)血管内侧视光声导管[46]
Fig. 2. Photoacoustic imaging systems. (a) Transmission-mode OR-PAM system; (b) reflection-mode OR-PAM system; (c) AR-PAM system with dark-field illumination; (d) PACT system with ring-shaped UTA; (e) PACT system with linear UTA; (f) PACT system with hemispherically shaped UTA; (g) PACT system based on 2D Fabry-Perot interferometer acoustic sensor; (h) side-viewing intravascular PA catheter[46]
PAM采用经处理后的光波聚焦照射组织体,聚焦的超声探测器接收组织内的超声信号,完整的成像需进行逐点扫描,因此成像结果无需算法进行图像重建。PAM依成像系统分辨率的不同分为光学分辨率光声显微镜(OR-PAM)[47]和声学分辨率光声显微镜(AR-PAM)[48]。当PAM的激光聚焦焦斑小于超声聚焦焦斑时,称之为OR-PAM,其中
光声内窥镜(PAE)是近几年发展起来用于人体内部器官疾病诊断的成像系统。以光声显微成像技术为基础发展的光声内窥主要应用于人体内血管[
3 光声成像在早期癌症诊断中应用
光声成像利用光声效应在成像分辨率上打破传统纯光学成像技术因组织散射导致的分辨率低的限制,为深层肿瘤的精细结构提供高对比度成像。通过区别组织的不同光学特性参数以及定量监测病变组织血氧饱和度、黏弹性等性质实现对离体与在体肿瘤的多尺度成像[53-56],为癌症诊断提供解剖学、功能性和代谢信息,继而实现癌症的早期诊断[57-58]。
3.1 定性化学参量癌症应用研究
肿瘤内不同组织的光学特性参数与其化学成分密切相关。各化学成分的吸收波长差异促使光声成像选择特定的波长对肿瘤内光声对比剂进行激发,从而为癌症诊断提供解剖学、形态学、分子学、基因学等信息。肿瘤内有许多新生血管生成[59],利用可见光波段作为光声激发源对血管内血红蛋白进行成像,即可获得组织内血管的高对比度影像,从而监测组织内脉管系统以及药物治疗后肿瘤血管的变化[60-61]。黑色素瘤是皮肤肿瘤中恶性程度最高的肿瘤,且容易发生转移。光声成像可以检测肿瘤内源性黑色素来实现皮肤黑色素瘤的诊断[62]、监测黑色素瘤的生长及其转移[63-64]。
细胞核作为控制DNA复制、 RNA合成以及核糖体装备[65]的细胞器,病理学认为细胞核的重叠和尺寸扩大是癌症的标志,并将细胞核形态的变化用于确定癌症分期以及预后评估标准[66]。DNA和RNA对紫外波段有强吸收效应,紫外光声显微成像可以获得肿瘤细胞核形态、尺寸和分布信息。人体前列腺腺癌细胞核核酸紫外光声光谱与Partin等[67]分级相关性达到0.85以上。手术切除未经处理以及染色的人体乳腺肿瘤利用透射式紫外光声显微进行细胞核尺寸、细胞堆积密度的特征诊断,对微小癌细胞群进行识别,成像结果与传统组织学结果比对具有高相关性[30]。癌组织结构,肿瘤边缘和正常组织的基本特征、浸润导管癌与原位导管癌在紫外光声显微镜下成像结果与组织染色后在显微镜下观察的形态一致,如
![乳腺癌的光声成像[30]。(a)固定未处理的乳腺癌UV-PAM图;(b)经过切片和染色处理的乳腺组织与(a)中相同区域的H&E染色的组织学图像;(c)(d)对应(a)(b)中的红色虚线区域放大后的UV-PAM和H&E染色图像;(e)(f)对应(a)(b)中的黄色虚线区域放大后的UV-PAM和H&E图像;(g)放大(a)中橙色虚线区域的UV-PAM图像](/richHtml/lop/2019/56/7/070001/img_3.jpg)
图 3. 乳腺癌的光声成像[30]。(a)固定未处理的乳腺癌UV-PAM图;(b)经过切片和染色处理的乳腺组织与(a)中相同区域的H&E染色的组织学图像;(c)(d)对应(a)(b)中的红色虚线区域放大后的UV-PAM和H&E染色图像;(e)(f)对应(a)(b)中的黄色虚线区域放大后的UV-PAM和H&E图像;(g)放大(a)中橙色虚线区域的UV-PAM图像
Fig. 3. Photoacoustic imaging of breast tumor[30]. (a) UV-PAM image of fixed, unprocessed breast tumor; (b) H&E-stained histologic image of same area shown in Fig. (a) acquired after sectioning and staining breast tissue; (c)(d) zoomed UV-PAM and H&E-stained images of red dashed regions in Fig. (a) and (b), respectively; (e)(f) zoomed UV-PAM and H&E images of yellow dashed regions in Fig. (a) and (b), respectively; (g) zoomed UV-PAM image of orange dashed region in Fig. (a)
外源光声造影剂可以增强图像对比度从而提高光声成像技术对深层肿瘤影像的敏感性。外源性光声造影剂主要利用配体和受体机制,通过生物化学等方法将分子探针(如抗体、多肽、DNA、RNA等生物大分子)和光声吸收体(如染料分子、纳米金、纳米银、碳纳米材料等)结合起来注入生物体。分子探针与肿瘤内靶向分子作用使得光声吸收体在靶向分子周围聚集,再通过光声成像技术获取光声吸收体在生物体内分布来反映肿瘤内靶向分子的分布情况,从而实现肿瘤的检测。例如,近红外吸收染料IRDye800因其吸收波峰在800 nm而用于胶质母细胞瘤[68]以及乳腺癌[69]的检测。金纳米粒子表面具有等离子体共振效应而广泛应用于黑色素瘤、鳞状细胞癌、前列腺癌、结肠癌、乳腺癌等的靶向检测[70-73]。银纳米粒子因表面效应、量子尺寸效应等而被应用于胰腺癌[74]等的检测。碳纳米材料经常作为基因和药物递送的载体,其纳米探针可实现胶质母细胞瘤、胃癌等的检测[75-76]。
3.2 定量化学参量的癌症应用研究
过量的氧燃烧是癌症诊断和治疗的标准[77]。由于基因突变导致的癌细胞过度增值[78]改变氧及营养物质的重新分配,从而导致血红蛋白特征代谢发生变化[79]。大量研究表明,肿瘤内富含血管,而血液中氧合血红蛋白和脱氧血红蛋白的吸收光谱不同(
式中:
式中:
免疫组织化学研究发现,恶性肿瘤脉管系统比正常组织更密集且无规律性,高密度的血管增强了肿瘤和正常组织光声成像对比度,从而提供组织内血管生成或脉管系统发生改变的信息以及安全无创地监测肿瘤不同阶段血液含氧量和肿瘤缺氧情况[81-82]。光声成像选择特定的波长去激发肿瘤可以获得血红蛋白的特征信息。多波长光声成像系统检测组织中总血红蛋白浓度和氧合血红蛋白的微小变化来获得肿瘤血红蛋白定量功能信息[83],实现肿瘤鉴定及肿瘤含氧量监测。光声与超声联合成像可以测量小鼠内肝细胞癌血氧饱和度以及血液灌注率,从而实现肝细胞癌的索拉菲尼治疗情况监测以及预测癌进展情况[84]。多光谱光声成像系统对急性淋巴细胞白血病小鼠的股骨骨髓血氧饱和度进行测量,结果表明股骨的血氧饱和度受白血病进展的影响,从而监测白血病发展情况[85]。相位滤波微分光声雷达(PAR)成像技术利用相位调制的两个激光波长抑制背景吸收,同时放大光声信号的差异,通过调节裸鼠大腿内的头颈癌肿瘤光声信号的幅度比和相移定量测量组织内血氧浓度从而实现癌症筛查。
![光声成像测量得到的肿瘤内的血氧浓度。(a)两个单波长振幅PAR技术;(b)双波长振幅微分PAR成像技术;(c)两个单波长相位滤波微分PAR技术;(d)双波长相位滤波微分PAR技术[80]](/richHtml/lop/2019/56/7/070001/img_5.jpg)
图 5. 光声成像测量得到的肿瘤内的血氧浓度。(a)两个单波长振幅PAR技术;(b)双波长振幅微分PAR成像技术;(c)两个单波长相位滤波微分PAR技术;(d)双波长相位滤波微分PAR技术[80]
Fig. 5. Oxygenation levels within tumor using photoacoustic imaging. (a) Two single-wavelength PAR amplitude technique; (b) two-wavelength differential PAR amplitude technique; (c) two single-wavelength phase-filtered differential PAR technique; (d) two-wavelength phase-filtered differential PAR technique[80]
3.3 黏弹参量的癌症应用研究
黏弹性质是与材料的热力学密切相关的物理性质,生物组织黏弹系数发生改变与组织病变有关[88]。组织的黏弹性系数是反映组织、器官代谢以及生理功能状态的重要参数,进行组织黏弹性系数的监测对研究生物组织的生理变化以及疾病诊疗具有重要意义[89]。由于组织的黏弹性质,光声信号相对于激励源会发生相位延迟,不同组织或者组织发生病变导致组织的黏弹系数发生改变从而导致光声信号产生不同的相位延迟,因此利用光声信号的相位延迟时间获得组织体的光声黏弹性成像,可判断组织的病变情况[90]。
光声成像对肿瘤进行诊断,可同时获得肿瘤光声信号的振幅(光学吸收强弱)以及相位延迟(黏弹性)信息,从而实现肿瘤筛查的特异性诊断[91]。光声黏弹成像与内窥镜相结合形成的光声黏弹内窥镜可应用于早期食道疾病诊断,尤其为肿瘤诊断提供生物力学信息以及形态特征的细微变化,从而提高肿瘤早期诊断率[92]。
![肿瘤的光声黏弹性成像。(a)肿瘤的光吸收图像;(b)肿瘤的黏弹性成像;(c)标记的肿瘤区域与正常组织的PA信号的平均幅度和相位延迟[91]](/richHtml/lop/2019/56/7/070001/img_6.jpg)
图 6. 肿瘤的光声黏弹性成像。(a)肿瘤的光吸收图像;(b)肿瘤的黏弹性成像;(c)标记的肿瘤区域与正常组织的PA信号的平均幅度和相位延迟[91]
Fig. 6. Photoacoustic viscoelastic imaging of tumor. (a) Optical absorption image of tumor; (b) viscoelasticity image of tumor; (c) averaged amplitude and phase delay of PA signal from marked tumor region and normal tissue [91]
3.4 声学功率谱参量的癌症应用研究
近年来由于光声成像在各领域的广泛应用,研究者对光声信号的研究热潮主要集中在可以获得组织体的结构与功能信息的时域光声光谱分析。为获得组织的亚波长微结构特性,观察细胞形态以及细胞分布密集程度[94-95]等,研究者根据声波的特性对光声信号进行频谱分析。研究表明,组织声学功率谱与组织内微结构的尺寸存在确定性的对应关系,组织声学功率谱与时域光声信号满足
式中:
式中:
式中:
![前列腺肿瘤的光声功率谱成像[100]。(a)肿瘤和正常组织在感兴趣区域的超声成像;(b) PA图像与超声图像叠加后的中频带拟合结果;(c) PA图像与超声图像叠加后的斜率;(d) PA图像与超声图像叠加后的截距](/richHtml/lop/2019/56/7/070001/img_7.jpg)
图 7. 前列腺肿瘤的光声功率谱成像[100]。(a)肿瘤和正常组织在感兴趣区域的超声成像;(b) PA图像与超声图像叠加后的中频带拟合结果;(c) PA图像与超声图像叠加后的斜率;(d) PA图像与超声图像叠加后的截距
Fig. 7. Photoacoustic power spectral imaging of prostate tumors[100]. (a) Ultrasonic imaging in region of interests for tumor and normal tissue; (b) mid-band fitting result after superposition of PA image and ultrasonic image; (c) slope after superposition of PA image and ultrasonic image; (d) intercept after superposition of PA image and ultrasonic image
4 光声成像的临床潜在应用研究
光声成像在临床基础研究中已实现对人体乳腺癌的血管造影[102]以及为乳腺影像报告和数据系统(BI-RADS)分类为4a和4b的患者进一步诊断肿瘤的良恶性[103-104],提高了乳腺肿瘤检测特异性,减少患者肿瘤活检次数以及短期随访的活检次数。光声成像系统还可用于引导乳腺癌前哨淋巴结活检,避免传统活检造成大面积的创伤[105-106]。光声成像不仅应用于早期癌症检测,还应用在心脑血管泌尿系统心肌缺血检测等方面。光声成像技术可以获得冠状动脉信息[107]以及评估急性心肌缺血的位置和缺血情况[108],并为急性心脑血管患者提供介入诊断与治疗[109]。实时对动脉闭塞时的脑功能情况[110]、脑部物质代谢[111]、癫痫病发作以及传播过程中脑部变化[112]进行监测,实时掌握病情发生发展情况。光声成像可观察诊断病变膀胱组织以及膀胱癌侵袭程度[113]、观察膀胱输尿管反流[114]等。光声成像与光动力疗法以及光热疗法相结合,可以监测肿瘤治疗结果与复发情况[115-116],监测光热疗法期间组织温度变化[117],实时控制和反馈肿瘤治疗情况[118]。
肿瘤转移成为定位癌肿位置的一大难题。多光谱光声成像技术以其高对比度、高分辨率、高穿透深度以及高检测特异性等优势成为对转移癌肿进行定位的研究热点[119],其可实时检测淋巴结中黑色素瘤转移[120-121]等。除此之外,光声成像技术还可应用于原发性肝癌边界界定[122]、检测卵巢中类似黄体的血管性卵巢组织[123]、皮肤色素沉着检测[124]、眼部疾病诊断[125]等预临床医学应用。
5 结束语
对癌肿瘤进行准确定位和表征是提供及时干预和有效治疗的关键。当前医学影像技术难以实现体内微小肿瘤的检测,尤其是检测早期肿瘤、肿瘤转移、肿瘤良恶性分辨、肿瘤代谢情况等。光声成像技术使用脉冲激光诱导部分组织热弹膨胀产生可由超声探测器检测的超声信号,而不使用有电离辐射作用的组织内在发色团或外源性造影剂进行成像,以一种非电离、高对比度、高分辨率、高穿透深度、不同组织成像特征明显的成像方式对老鼠、兔子、犬类等小动物身上各组织器官进行非侵入式、无损成像,继而对人体组织进行离体与部分在体研究,实现癌诊断、肿瘤转移监测、肿瘤治疗监测、脑损伤诊断、血管结构与功能诊断。但由于光在组织中传播过程的散射作用以及组织对光子吸收作用,使得光声成像技术在软组织中成像深度受到限制。若采用波前工程技术等来补偿光在组织中传播的散射效应,则有望实现光声成像技术在未来临床上早期肿瘤的检测。
光声成像技术能同时从生物体组织的生化成分、力学特性等方面提供生物体系统的解剖学、分子学、基因学、功能性、代谢等多维信息,为深层组织提供毫米乃至纳米量级的成像分辨率,为生物组织从亚细胞结构至细胞器官病变诊断提供多尺度的成像信息,为临床医学疾病诊断提供从宏观到微观、从单一参量到多尺度、从结构到功能、从定性到定量的成像信息。随着成像设备、成像算法、信号增强等方面的不断完善与发展,光声成像正在向多模态、多功能、诊疗一体化方向发展以期满足临床生物医学应用的要求。光声成像以一种低成本、便携、无损、检测特异性高等成像方式将为人类疾病诊断与治疗、药物研制等提供多维度的影像信息,从而改善临床影像的不足与疾病预后情况,提高人类生活质量。
目前光声成像在癌症诊疗应用仍处于基础实验研究至临床应用的转化阶段,该阶段需要更多的理论与实验经验为光声成像向临床应用转化奠定基础。光声成像因光在组织中传播过程的散射作用致使光声成像不便用于全身或深层病变组织成像。由于光声成像扫描条件比较繁琐,超声换能器带宽无法满足从低频到高频的采集范围,数据处理软件还不够完善等限制,应用于早期癌症筛查和癌症治疗过程中动态监测疗效的低成本、无损、高性能的实时临床光声成像系统仍需进一步的研发与实践。
[1] StewartB, Wild CP. World cancer report 2014[R]. Geneva: World Health Organization, 2015.
[2] Siegel R L, Miller K D, Jemal A. Cancer statistics, 2017[J]. CA: A Cancer Journal for Clinicians, 2017, 67(1): 7-30.
[3] Bell A G. On the production and reproduction of sound by light[J]. American Journal of Science, 1880, 20: 305- 324.
[11] Xu M H, Wang L V. Photoacoustic imaging in biomedicine[J]. Review of Scientific Instruments, 2006, 77(4): 041101.
[22] Gottschalk S. FelixFehm T, LuísDeán-Ben X, et al. Noninvasive real-time visualization of multiple cerebral hemodynamic parameters in whole mouse brains using five-dimensional optoacoustic tomography[J]. Journal of Cerebral Blood Flow & Metabolism, 2015, 35(4): 531-535.
[37] Andreev V G, Karabutov A A, Solomatin S V, et al. Optoacoustic tomography of breast cancer with arc-array transducer[J]. Proceedings of SPIE, 2000, 3916: 36-48.
[38] Meiburger M, Nam Y, Chung E, et al. Skeletonization algorithm-based blood vessel quantification using in vivo 3D photoacoustic imaging[J]. Physics in Medicine and Biology, 2016, 61(22): 7994-8009.
[39] Ding T, Ren K, Vallélian S. A one-step reconstruction algorithm for quantitative photoacoustic imaging[J]. Inverse Problems, 2015, 31(9): 095005.
[40] Hoelen C G A. A new theoretical approach to photoacoustic signal generation[J]. The Journal of the Acoustical Society of America, 1999, 106(2): 695-706.
[41] 邵惠民. 数学物理方法[M]. 第2版. 北京: 科学出版社, 2010.
Shao HM. Mathematical physical method[M]. 2rd ed. Beijing: Science Press, 2010.
[42] 梁昆淼. 数学物理方法[M]. 第4版. 北京: 高等教育出版社, 2010.
Liang KM. Mathematical physical method[M]. 4th ed. Beijing: Higher Education Press, 2010.
[43] Kolkman R G M, Hondebrink E, et al. . Photoacoustic determination of blood vessel diameter[J]. Physics in Medicine and Biology, 2004, 49(20): 4745-4756.
[44] Paltauf G, Schmidt-Kloiber H, Frenz M. Photoacoustic waves excited in liquids by fiber-transmitted laser pulses[J]. The Journal of the Acoustical Society of America, 1998, 104(2): 890-897.
[45] Paltauf G, Schmidt-Kloiber H. Pulsed optoacoustic characterization of layered media[J]. Journal of Applied Physics, 2000, 88(3): 1624-1631.
[46] Wang L V, Yao J J. A practical guide to photoacoustic tomography in the life sciences[J]. Nature Methods, 2016, 13(8): 627-638.
[47] Hu S, Maslov K, Wang L V. Second-generation optical-resolution photoacoustic microscopy with improved sensitivity and speed[J]. Optics Letters, 2011, 36(7): 1134-1136.
[48] Vienneau E, Liu W, Yao J J. Dual-view acoustic-resolution photoacoustic microscopy with enhanced resolution isotropy[J]. Optics Letters, 2018, 43(18): 4413-4416.
[49] Leng X D, Chapman W, Rao B. et al. Feasibility of co-registered ultrasound and acoustic-resolution photoacoustic imaging of human colorectal cancer[J]. Biomedical Optics Express, 2018, 9(11): 5159-5172.
[50] Yuan Y, Yang S H, Xing D. Preclinical photoacoustic imaging endoscope based on acousto-optic coaxial system using ring transducer array[J]. Optics Letters, 2010, 35(13): 2266-2268.
[51] Wu H Q, Li Z R, Liu L T, et al. Photoacoustic imaging of early gastric cancer diagnosis based on long focal area ultrasound transducer[J]. Journal of Physics: Conference Series, 2017, 844: 012051.
[52] 彭东青, 谢文明, 吴淑莲, 等. 基于柱弥散光源体内辐照的前列腺扫描光声成像仿体实验[J]. 物理学报, 2015, 64(20): 207801.
Peng D Q, Xie W M, Wu S L, et al. Phantom experimental photoacoustic scanning imaging of prostate based on internal light irradiation using cylindrical diffusing source[J]. Acta Physica Sinica, 2015, 64(20): 207801.
[53] Fakhrejahani E, Torii M, Kitai T, et al. Clinical report on the first prototype of a photoacoustic tomography system with dual illumination for breast cancer imaging[J]. PLoS One, 2015, 10(10): e0139113.
[54] Tian C, Qian W, Shao X, et al. Photoacoustic imaging: plasmonic nanoparticles with quantitatively controlled bioconjugation for photoacoustic imaging of live cancer cells[J]. Advanced Science, 2016, 3(12): 1600237.
[55] Priya M. Rao B S S, Chandra S, et al. Photoacoustic spectroscopy based investigatory approach to discriminate breast cancer from normal: a pilot study[J]. Proceedings of SPIE, 2016, 9689: 968943.
[56] Chen YS, YeagerD, Emelianov SY. Photoacoustic imaging for cancer diagnosis and therapy guidance[M]. Amsterdam: Elsevier, 2014: 139- 158.
[57] Valluru K S, Willmann J K. Clinical photoacoustic imaging of cancer[J]. Ultrasonography, 2016, 35(4): 267-280.
[58] Lin L, Hu P, Shi J H, et al. Clinical photoacoustic computed tomography of the human breast in vivo within a single breath hold[J]. Proceedings of SPIE, 2018, 10494: 104942X.
[59] Triratanachat S, Niruthisard S, Trivijitsilp P, et al. Angiogenesis in cervical intraepithelial neoplasia and early-staged uterine cervical squamous cell carcinoma: clinical significance[J]. International Journal of Gynecological Cancer, 2006, 16(2): 575-580.
[60] Toi M, Asao Y. MatsumotoY, et al. Visualization of tumor-related blood vessels in human breast by photoacoustic imaging system with a hemispherical detector array[J]. Scientific Reports, 2017, 7: 41970.
[61] Bohndiek S E, Sasportas L S. MacHtaler S, et al. Photoacoustic tomography detects early vessel regression and normalization during ovarian tumor response to the antiangiogenic therapy trebananib[J]. Journal of Nuclear Medicine, 2015, 56(12): 1942-1947.
[62] Breathnach A, Concannon E, Dorairaj J J, et al. Preoperative measurement of cutaneous melanoma and nevi thickness with photoacoustic imaging[J]. Journal of Medical Imaging, 2018, 5(1): 015004.
[63] Breathnach A, Concannon L, Aalto L, et al. Assessment of cutaneous melanoma and pigmented skin lesions with photoacoustic imaging[J]. Proceedings of SPIE, 2015, 9303: 930303.
[64] Lavaud J, Henry M, Coll J L, et al. Exploration of melanoma metastases in mice brains using endogenous contrast photoacoustic imaging[J]. International Journal of Pharmaceutics, 2017, 532(2): 704-709.
[65] ZimmermannA. Nucleus, nuclear structure, and nuclear functions: pathogenesis of nuclear abnormalities in cancer[M]. Cham: Springer International Publishing, 2016: 3071- 3087.
[66] Singh N, Gilks C B. The changing landscape of gynaecological cancer diagnosis: implications for histopathological practice in the 21st century[J]. Histopathology, 2017, 70(1): 56-69.
[67] Partin A W, Kattan M W. Subong E N P, et al. Combination of prostate-specific antigen, clinical stage, and gleason score to predict pathological stage of localized prostate cancer[J]. JAMA, 1997, 277(18): 1445-1451.
[68] Attia A B E, Ho C J H, Chandrasekharan P, et al. . Multispectral optoacoustic and MRI coregistration for molecular imaging of orthotopic model of human glioblastoma[J]. Journal of Biophotonics, 2016, 9(7): 701-708.
[69] Stantz K M, Cao M S, Liu B, et al. Molecular imaging of neutropilin-1 receptor using photoacoustic spectroscopy in breast tumors[J]. Proceedings of SPIE, 2010, 7564: 75641O.
[70] Weber J, Beard P C, Bohndiek S E. Contrast agents for molecular photoacoustic imaging[J]. Nature Methods, 2016, 13(8): 639-650.
[71] Liu C, Li S Y, Gu Y J, et al. Multispectral photoacoustic imaging of tumor protease activity with a gold nanocage-based activatable probe[J]. Molecular Imaging and Biology, 2018, 20(6): 919-929.
[72] Li W W, Chen X Y. Gold nanoparticles for photoacoustic imaging[J]. Nanomedicine, 2015, 10(2): 299-320.
[73] Balasundaram G. Ho C J H, Li K, et al. Molecular photoacoustic imaging of breast cancer using an actively targeted conjugated polymer[J]. International Journal of Nanomedicine, 2015, 10: 387.
[74] Wilson KE, Valluru KS, Willmann JK. Nanoparticles for photoacoustic imaging of cancer[M]. Cham: Springer International Publishing, 2016: 315- 335.
[75] Sajid M I, Jamshaid U, Jamshaid T, et al. Carbon nanotubes from synthesis to in vivo biomedical applications[J]. International Journal of Pharmaceutics, 2016, 501(1/2): 278-299.
[76] Kumar S, Rani R, Dilbaghi N, et al. Carbon nanotubes: a novel material for multifaceted applications in human healthcare[J]. Chemical Society Reviews, 2017, 46(1): 158-196.
[77] Vaupel P, Mayer A. The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities[J]. Antioxidants & Redox Signaling, 2015, 22(10): 878-880.
[78] Zhang LY. Identification and characterization of tumor suppressor gene and cancer stemness gene in esophageal squamous cell carcinoma[D]. Hong Kong: The University of Hong Kong Libraries, 2015.
[79] ZhangM, Liu CM, Zhang ZH, et al. A new flavonoid regulates angiogenesis and reactive oxygen species production[M]. New York: Springer, 2014: 149- 155.
[80] Dovlo E, Lashkari B, Sean Choi S, et al. Quantitative phase-filtered wavelength-modulated differential photoacoustic radar tumor hypoxia imaging toward early cancer detection[J]. Journal of Biophotonics, 2017, 10(9): 1134-1142.
[81] Lin R, Chen J, Wang H. et al. Longitudinal label-free optical-resolution photoacoustic microscopy of tumor angiogenesis in vivo[J]. Quantitative Imaging in Medicine and Surgery, 2015, 5(1): 23.
[82] Gerling M, Zhao Y, Nania S, et al. Real-time assessment of tissue hypoxia in vivo with combined photoacoustics and high-frequency ultrasound[J]. Theranostics, 2014, 4(6): 604-613.
[83] Paproski R J, Heinmiller A, Wachowicz K, et al. Multi-wavelength photoacoustic imaging of inducible tyrosinase reporter gene expression in xenograft tumors[J]. Scientific Reports, 2015, 4: 5329.
[84] Naser M A, Munoz N. Sampaio D R T, et al. Imaging biomarker development based on microbubble perfusion and oxygen saturation in a rat model of liver cancer[J]. Proceedings of SPIE, 2018, 10580: 1058007.
[85] Wood C, Harutyunyan K. Cerda J D L, et al. Assessment of blood oxygen saturation using spectroscopic photoacoustic imaging as a biomarker for disease progression in a small-animal leukemia model[J]. Proceedings of SPIE, 2018, 10580: 105800W.
[86] Gray L H, Steadman J M. Determination of theoxyhaemoglobin dissociation curves for mouse and rat blood[J]. The Journal of Physiology, 1964, 175(2): 161-171.
[87] Siphanto R I, Thumma K K. Kolkman R G M, et al. Serial noninvasive photoacoustic imaging of neovascularization in tumor angiogenesis[J]. Optics Express, 2005, 13(1): 89-95.
[88] Wang S, Larin K V. Optical coherence elastography for tissue characterization: a review[J]. Journal of Biophotonics, 2015, 8(4): 279-302.
[89] 王金华. 激光散斑组织弹性成像初步研究[D]. 武汉: 华中科技大学, 2014.
Wang JH. Preliminary study on laser speckle tissue elastography[D]. Wuhan: Huazhong University of Science and Technology, 2014.
[90] Glatz T, Scherzer O, Widlak T. Texture generation for photoacoustic elastography[J]. Journal of Mathematical Imaging and Vision, 2015, 52(3): 369-384.
[91] Zhao Y, Yang S H, Chen C G, et al. Simultaneous optical absorption and viscoelasticity imaging based on photoacoustic lock-in measurement[J]. Optics Letters, 2014, 39(9): 2565-2568.
[92] Jin D Y, Yang F, Chen Z J, et al. Biomechanical and morphological multi-parameter photoacoustic endoscope for identification of early esophageal disease[J]. Applied Physics Letters, 2017, 111(10): 103703.
[93] Mallidi S, Luke G P, Emelianov S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance[J]. Trends in Biotechnology, 2011, 29(5): 213-221.
[94] Biswas D, Gorey A. Chen G C K, et al. Investigation of diseases through red blood cells' shape using photoacoustic response technique[J]. Proceedings of SPIE, 2015, 9322: 93220K.
[95] Saha R K, Fadhel M N, Lawrence A, et al. Rapid computation of photoacoustic fields from normal and pathological red blood cells using a Green's function method[J]. Proceedings of SPIE, 2017, 10064: 100644U.
[96] Rabiner L R, Gold B. Theory and application of digital signal processing[J]. Englewood Cliffs, NJ, Prentice-Hall, Inc., 1975, 777.
[97] Cheong C, Joseph P, Lee S. High frequency formulation for the acoustic power spectrum due to cascade-turbulence interaction[J]. The Journal of the Acoustical Society of America, 2006, 119(1): 108-122.
[98] Sinha S, Rao N A, Chinni B K, et al. Evaluation of frequency domain analysis of a multiwavelength photoacoustic signal for differentiating malignant from benign and normal prostates[J]. Journal of Ultrasound in Medicine, 2016, 35(10): 2165-2177.
[99] Nandy S, Mostafa A, Hagemann I S. et al. Evaluation of ovarian cancer: initial application of coregistered photoacoustic tomography and US[J]. Radiology, 2018, 289(3): 740-747.
[100] Kumon R E, Deng C X, Wang X D. Frequency-domain analysis of photoacoustic imaging data from prostate adenocarcinoma tumors in a murine model[J]. Ultrasound in Medicine & Biology, 2011, 37(5): 834-839.
[101] Wang S H, Tao C, Yang Y Q, et al. Theoretical and experimental study of spectral characteristics of the photoacoustic signal from stochastically distributed particles[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2015, 62(7): 1245-1255.
[102] Lin L, Hu P, Shi J H, et al. Single-breath-hold photoacoustic computed tomography of the breast[J]. Nature Communications, 2018, 9: 2352.
[103] Neuschler E I, Butler R, Young C A, et al. A pivotal study of optoacoustic imaging to diagnose benign and malignant breast masses: a new evaluation tool for radiologists[J]. Radiology, 2018, 287(2): 398-412.
[104] Menezes G L G, Pijnappel R M, Meeuwis C, et al. . Downgrading of breast masses suspicious for cancer by using optoacoustic breast imaging[J]. Radiology, 2018, 288(2): 355-365.
[105] Garcia-Uribe A, Erpelding T N, Krumholz A, et al. Dual-modality photoacoustic and ultrasound imaging system for noninvasive sentinel lymph node detection in patients with breast cancer[J]. Scientific Reports, 2015, 5: 15748.
[106] Li M C, Liu C B, Gong X J, et al. Linear array-based real-time photoacoustic imaging system with a compact coaxial excitation handheld probe for noninvasive sentinel lymph node mapping[J]. Biomedical Optics Express, 2018, 9(4): 1408-1422.
[107] Daeichin V, Chen C, Ding Q, et al. A broadband polyvinylidene difluoride-based hydrophone with integrated readout circuit for intravascular photoacoustic imaging[J]. Ultrasound in Medicine & Biology, 2016, 42(5): 1239-1243.
[108] Li Z F, Li H, Chen H Y, et al. In vivo determination of acute myocardial ischemia based on photoacoustic imaging with a focused transducer[J]. Journal of Biomedical Optics, 2011, 16(7): 076011.
[109] Piao Z L, Ma T, Qu Y Q, et al. High speed intravascular photoacoustic imaging of atherosclerotic arteries[J]. Proceedings of SPIE, 2016, 9689: 968930.
[110] Kneipp M, Turner J, Hambauer S, et al. Functional real-time optoacoustic imaging of middle cerebral artery occlusion in mice[J]. PLoS One, 2014, 9(4): e96118.
[111] TangJ, ColemanJ, DaiX, et al. 3D photoacoustic tomography brain imaging in behaving animal[C]∥Optical Tomography and Spectroscopy Optical Society of America, 2016: OM2C. 3.
[112] Zhang Q Z, Liu Z, Carney P R, et al. Non-invasive imaging of epileptic seizures in vivo using photoacoustic tomography[J]. Physics in Medicine and Biology, 2008, 53(7): 1921-1931.
[113] Xie Z X, Roberts W, Carson P, et al. Evaluation of bladder microvasculature with high-resolution photoacoustic imaging[J]. Optics Letters, 2011, 36(24): 4815-4817.
[114] Kim C, Jeon M, Wang L V. Nonionizing photoacoustic cystography in vivo[J]. Optics Letters, 2011, 36(18): 3599-3601.
[115] Mallidi S, Watanabe K, Timerman D, et al. Prediction of tumor recurrence and therapy monitoring using ultrasound-guided photoacoustic imaging[J]. Theranostics, 2015, 5(3): 289-301.
[116] Ho C JH, BalasundaramG, DriessenW, et al. Photoacoustic diagnostic imaging of photodynamic therapeutic contrast agents[C]∥Biomedical Optics, 2014: BS4A. 5.
[117] Li Z F, Liu Y B, Li H, et al. Monitoring tissue temperature for photothermal cancer therapy based on photoacoustic imaging: a pilot study[J]. Proceedings of SPIE, 2013, 8582: 858209.
[118] Li Z F, Chen H Y, Zhou F F, et al. Interstitial photoacoustic sensor for the measurement of tissue temperature during interstitial laser phototherapy[J]. Sensors, 2015, 15(3): 5583-5593.
[119] Stoffels I, Morscher S, Helfrich I, et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging[J]. Science Translational Medicine, 2015, 7(317): 199.
[120] Langhout G C, Grootendorst D J, Nieweg O E, et al. Detection of melanoma metastases in resected human lymph nodes by noninvasive multispectral photoacoustic imaging[J]. International Journal of Biomedical Imaging, 2014, 2014: 163652.
[121] Neuschmelting V, Lockau H, Ntziachristos V, et al. Lymph node micrometastases and in-transit metastases from melanoma: in vivo detection with multispectral optoacoustic imaging in a mouse model[J]. Radiology, 2016, 280(1): 137-150.
[122] 关天培, 方驰华. 光声成像技术及其在原发性肝癌边界界定中的应用[J]. 中华肝脏外科手术学电子杂志, 2016, 5(2): 65-67.
Guan T P, Fang C H. Photoacoustic imaging technique and its application in the demarcation of primary liver cancer[J]. Chinese Journal of Hepatic Surgery, 2016, 5(2): 65-67.
[123] Aguirre A, Guo P Y, Gamelin J, et al. Coregistered three-dimensional ultrasound and photoacoustic imaging system for ovarian tissue characterization[J]. Journal of Biomedical Optics, 2009, 14(5): 054014.
[124] 邢达, 王雅婷, 许栋, 等. 一种基于光声原理的皮肤色素沉着成像装置:104146685A[P].2014-11-19.
XingD, Wang YT, XuD, et al. A skin pigmentation imaging device based on photoacoustic principle:104146685A[P]. 2014-11-19.
[125] Zackrisson S, Gambhir S S. Light in and sound out: emerging translational strategies for photoacoustic imaging[J]. Cancer Research, 2014, 74(4): 979-1004.
Article Outline
吴华钦, 王昊宇, 谢文明, 李志芳, 吴淑莲, 李晖. 光声成像技术在早期癌症检测治疗中的潜在应用[J]. 激光与光电子学进展, 2019, 56(7): 070001. Huaqin Wu, Haoyu Wang, Wenming Xie, Zhifang Li, Shulian Wu, Hui Li. Potential Applications of Photoacoustic Imaging in Early Cancer Diagnosis and Treatment[J]. Laser & Optoelectronics Progress, 2019, 56(7): 070001.
![血红蛋白吸收光谱[83]](/richHtml/lop/2019/56/7/070001/img_4.jpg)