Rb掺杂对K-Cs-Sb阴极材料光电性质的影响
Alkali antimonide photocathodes are widely used in many fields such as radiation detection, photon counting, and accelerator electron source due to their advantages of high quantum efficiency, long lifespan, short response time, and low preparation cost. Since K2CsSb bi-alkali photocathode has high photosensitivity ranging from 300 nm to 650 nm, it is often used as the key component of large-area microchannel plate photomultiplier tube and dynode photomultiplier tube. K-Cs-Rb-Sb tri-alkali photocathodes may exhibit more outstanding performance in spectral response enhancement and thermionic emission suppression compared to conventional K2CsSb bi-alkali photocathode. So far, there have been little theoretical researches on K-Cs-Rb-Sb tri-alkali photocathodes. Due to the difficulty in controlling the stoichiometric ratio of alkali metal elements during the actual preparation processes of K-Cs-Rb-Sb photocathodes, and in fact K-Cs-Rb-Sb tri-alkali photocathodes with different stoichiometric ratios have different photoemission properties, it is necessary to analyze the mechanism of Rb doping leading to different photocathode properties from the atomic and electronic perspective, thereby providing theoretical guidance for designing excellent alkali antimonide photocathodes.
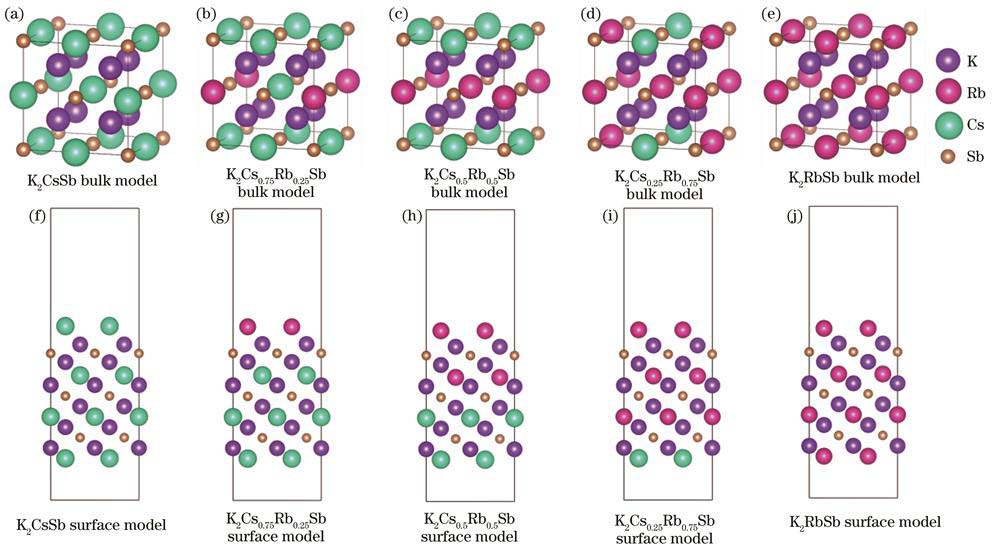
The K2Cs2-xRbxSb bulk models and the (111)-oriented surface models with different Cs/Rb ratios corresponding to K2CsSb,K2Cs0.75Rb0.25Sb,K2Cs0.5Rb0.5Sb,K2Cs0.25Rb0.75Sb,and K2RbSb were established. The K2CsSb unit cell belongs to the DO3 cubic structure with a lattice constant of 0.8615 nm, and the space group is Fm-3m. According to the number of Cs atoms in K2CsSb replaced by Rb atoms, the lattice constants of several K-Cs-Rb-Sb bulk models after atom replacements were obtained by Vegard law. On the basis of the K2CsSb (111) Cs-terminated surface, six, eight, twelve, and sixteen Cs atoms were replaced from top to bottom, to obtain the K-Cs-Rb-Sb(111) surface models with different Cs/Rb ratios. To eliminate inter-layer interactions caused by the periodic mirror interaction between the surface slabs, a vacuum layer of 2 nm was set along the z-axis, including an upper vacuum layer with a thickness of 1.5 nm and a lower vacuum layer with a thickness of 0.5 nm. During the structural optimization process, the upper surface atoms with a thickness of 0.8 nm were allowed to fully relax, while the remaining atoms were constrained. The VASP software package using the first-principles calculation method based on the density functional theory was adopted. The projected augmented wave method was used as the pseudo potential, the generalized gradient approximation function proposed by Perdew-Burke-Ernzerhof was used to express the exchange correlation interaction, the plane wave expansion with a cut-off energy of 500 eV was used, and the conjugate gradient method was used to optimize the lattice constants and atom positions of the diverse models. The K-point grid in the Monkhorst-Pack form was set as 6×6×6 for bulk models and 6×6×1 for surface models, respectively.
The calculation results indicate that when Rb atoms replace Cs atoms in the K-Cs-Rb-Sb bulk models with different Cs/Rb ratios, the optical properties including reflectivity, refractive index, extinction coefficient, and absorption coefficient are hardly affected by Rb doping. This implies that the incorporation of Rb atoms has minimal impact on the optical properties of K2CsSb material. From the perspective of formation energy and formation enthalpy, all the K-Cs-Rb-Sb bulk models where Rb atoms replace K atoms have positive formation energies, and the corresponding formation enthalpies are larger than that of the K2CsSb model. This indicates that it is very difficult for K atoms to be replaced by Rb atoms in the preparation process of K-Cs-Rb-Sb tri-alkali photocathodes. At the same time, all K-Cs-Rb-Sb bulk models where Rb atoms replace Cs atoms have negative formation energies, and the corresponding formation enthalpies are less than that of the K2CsSb model, indicating that all the models where Rb atoms replace Cs atoms are easy to form with better thermodynamic stability. As the number of Rb atoms replacing Cs atoms increases, the formation energies and formation enthalpies gradually decrease. This means that in the presence of both Cs and Rb, the K2Cs0.25Rb0.75Sb model is the easiest to form and the most stable. All K-Cs-Rb-Sb bulk models exhibit the property of p-type semiconductor, and K2Cs0.25Rb0.75Sb has the smallest bandgap. For K-Cs-Rb-Sb surface models with different Cs/Rb ratios, the vacuum levels, surface energies, and electron effective masses gradually decrease. Among them, the K2Cs0.25Rb0.75Sb surface model has the smallest ionization energy, indicating that its electrons generated under external light excitation are more likely to transit from the valence band top to the conduction band bottom and move in the conduction band. This is beneficial for enhancing the spectral response of the photocathode and further improving the photoelectric conversion efficiency. Doping Rb element in K2CsSb can increase the work function of the surface model. On the whole, the K2CsRb0.250.75Sb (111) with a larger work function and surface can prevent the escape of some hot electrons while ensuring that a large number of photoelectrons can escape from the surface, in order to achieve the reduction of cathode dark current without reducing its quantum efficiency. In the surface model containing K, Cs, and Rb alkali metals, K2Cs0.25Rb0.75Sb has the highest conductivity, because the concentration of conduction band electrons gradually increases, and the effective mass of conduction band electrons in the surface model decreases as the number of Cs atoms replaced by Rb atoms increases.
When Rb atoms replace Cs atoms, Rb doping has little effect on the optical properties of K-Cs-Rb-Sb cathode materials. For K-Cs-Rb-Sb bulk models with different Cs/Rb ratios, K2Cs0.25Rb0.75Sb has the lower formation energy and formation enthalpy, indicating that it is easy to form under natural conditions and it is thermodynamically stable. For the surface models, K2Cs0.25Rb0.75Sb has the smaller surface energy and higher conductivity, as well as the smallest bandgap and ionization energy. Besides, the work function of K2Cs0.25Rb0.75Sb is larger than that of K2CsSb. Therefore, the K-Cs-Rb-Sb cathode with a Cs/Rb ratio (atomic number fraction) of 1∶3 is considered to be a stable photoemission material with high quantum efficiency, low dark current, and good conductivity. The research results can provide guidance for the preparation of high-performance K-Cs-Rb-Sb photocathodes. In the traditional K2CsSb photocathode preparation process, doping Rb elements can reduce the dark noise of the photomultiplier tube while maintaining a high level of quantum efficiency, thereby improving the detection sensitivity and accuracy of the device in practical applications.
韩允锋, 金睦淳, 任玲, 王兴超, 张锴珉, 刘晓荣, 钱芸生, 张益军. Rb掺杂对K-Cs-Sb阴极材料光电性质的影响[J]. 光学学报, 2024, 44(4): 0416001. Yunfeng Han, Muchun Jin, Ling Ren, Xingchao Wang, Kaimin Zhang, Xiaorong Liu, Yunsheng Qian, Yijun Zhang. Effect of Rb Doping on Photoelectric Properties of K-Cs-Sb Cathode Material[J]. Acta Optica Sinica, 2024, 44(4): 0416001.