基于核仁靶向碳点的双光子光动力疗法研究  下载: 939次特邀研究论文亮点文章
下载: 939次特邀研究论文亮点文章
Photodynamic therapy (PDT), which canablate cancer cells or diseased tissue by the generated reactive oxygen species (ROS) once the photosensitizers (PSs) are excited by light with specific wavelength, has attracted various attention in the last decades due to its unique advantages, including non-invasiveness, few side-effects, etc. The advancement of PDT has been significantly restricted by the penetration depth of the excitation light and sub-cellular organelles targeting capability. Here, an effective carbon dots (C-dots) photosensitizer with intrinsic nucleolus-targeting capability is synthesized, characterized, and employed for in vitro photodynamic anticancer therapy with enhanced treatment performance at a low dose of PS and light irradiation.
The optical system, which included a microscope and femtosecond laser, was designed for two-photon phototherapy. The nucleolus-targeted C-dots were synthesized using microwave heating. The characteristics of synthesized C-dots including particles size, absorption and emission, two-photon fluorescence, photobleaching, biocompatibility, etc., were measured by DLS measurement, UV-Visible spectrophotometer, fluorescence spectrometer, femtosecond laser, CCK-8 kit, respectively. The nucleolus-targeting capability of C-dots was investigated using fluorescence imaging. The HeLa cells were incubated with C-dots and irradiated with a femtosecond laser before cell viability was examined using Calcein-AM/PI staining and fluorescence imaging.
The microwave heating method selects citric acid and ethylenediamine as raw materials to synthesize the C-dots. The synthesized C-dots were studied using dynamic light scattering measurement, and the average size of the C-dots was approximately 1 nm [Fig. 2(a)]. The C-dots absorbed light in various wavelengths from 300 to 700 nm, with the main absorption peak at 360 nm and a shoulder peak at 450 nm [Fig. 2(b)]. The C-dots exhibited excitation-dependent emission [Fig. 2(c)] and significant two-photon fluorescence when exposed to femtosecond laser irradiation [Fig. 2(d)]. The ROS-generation capability of the C-dots in aqueous solutions was investigated using ABDA as the ROS indicator under white light irradiation (400-700 nm, 100 mW/cm2). The ABDA was almost decomposed after 10 min illumination [Fig. 2(e)], indicating the ROS-generation capability of the C-dots. The long-term stability and photostability of the C-dots were characterized by measuring the absorption spectra at different time points [Fig. 2(f)] and after continuous irradiation [Fig. 2(g)], respectively. The experimental results showed that the C-dots had good long-term stability and photostability. CCK-8 kits were used to evaluate the biocompatibility of the C-dots before undergoing in vitro photodynamic therapy. For 24 and 48 h, no significant difference existed between control cells and cells treated with the C-dots in the mass concentration range of 250-750 g/mL, indicating the excellent biocompatibility of the C-dots. The C-dots were treated with HeLa cells to investigate their intracellular position, followed by fluorescence imaging. The fluorescence signal of the C-dots was observed in some round areas, i.e., nucleoli. HeLa cells were co-stained with the C-dots and one commercial nucleolus imaging probe, SYTO RNASelect, to demonstrate the C-dots’ nucleolus-targeting capacity. Figure 3 showed the fluorescence of the C-dots completely overlapped with that of SYTO RNASelect, confirming that the C-dots could specifically stain the nucleolus. HeLa cells were cultured with/without the C-dots (500 g/mL) for 3 h before being exposed to femtosecond laser irradiation to study the two-photon PDT efficiency of the C-dots (740 nm, 28 mW). The treated cells were incubated for 4 h following the irradiation and stained with Calcein-AM and PI. Figure 4 showed that more cells were PI-positive with an increment of irradiation time. When the irradiation time reached 45 s, almost all cells were necrotic, suggesting the excellent cancer cell ablation potential of nucleolus-targeted two-photon photodynamic treatment. The identical irradiation did not result in necrosis in the absence of the C-dots, indicating that laser irradiation had no effect.
We designed and synthesized novel C-dots with intrinsic nucleolus-targeting and ROS-generation capabilities. The nucleolus-targeted two-photon PDT exhibits outstanding cancer cell ablation efficiency at a low dose of the C-dots and light irradiation because the C-dots generated ROS is positioned within the nucleolus, which is an efficient cancer therapy site. Additionally, the developed C-dots possess some unique advantages, including ultrasmall size, long-term stability, and excellent biocompatibility, making them promising for practical two-photon PDT applications.
1 引言
癌症是威胁人类健康的重大疾病之一,有效的肿瘤治疗是降低死亡率的关键。目前临床使用的肿瘤治疗方法主要包括手术切除、化疗和放疗等,但这些方法都有一定的局限性,同时也存在一定的副作用[1-2]和并发症[3-4]。在众多肿瘤治疗新方法中,光动力疗法(PDT)是一种极具潜力的治疗恶性肿瘤的新技术[5-7]。光动力疗法是利用无毒性光敏剂在特定波长光照射下产生的活性氧(ROS)与癌细胞内的生物分子发生氧化反应,产生细胞毒性,进而杀伤肿瘤细胞的一种治疗方法[8-9]。光动力疗法具有治疗精准、副作用小、非侵入等优点,自20世纪80年代首次应用于临床试验以来,得到了快速发展,特别是在肿瘤学[10-12]、眼科和皮肤科等领域发展迅猛。肿瘤的光动力治疗效果不仅取决于光敏剂产生活性氧的量,还取决于光敏剂的亚细胞定位[13-14]。
近年来的研究工作主要集中于研发具有高活性氧产生效率和亚细胞结构靶向能力的光敏剂。国内外研究团队研制了诸多高性能光敏剂[15-17],包括聚集诱导发光光敏剂、石墨烯量子点、金属-有机物框架量子点[18]、半导体纳米颗粒等[19-21],其中石墨烯量子点的活性氧产率高达1.3[21]。此外,靶向线粒体[22]、溶酶体[23]、内质网[24]、高尔基体[25]、核仁[26]等亚细胞靶点的光动力疗法已经得到了深入且充分的研究,其中核仁靶向的光动力疗法取得了良好的肿瘤治疗效果。
但由于能级的限制,几乎所有光敏剂的吸收波长都位于可见光波段[27]。由于可见光在生物组织中的穿透深度有限[28-29],光动力疗法在深层肿瘤组织及肿瘤内部产生的活性氧非常有限,因此,光动力疗法在临床中主要用于浅表肿瘤的治疗[5]。但是,深层肿瘤组织的光动力治疗技术仍是基础研究和临床应用需要攻克的技术难题,迫切需要开发出适用于深层肿瘤组织光动力治疗的新技术、新方法。自Prasad研究团队[30]于1997年提出双光子光动力疗法的概念以来,国内外多个研究机构致力于该领域的研究,开展了各具特色的研究工作,并取得了一系列具有较高影响力的原创性成果[31-35]。但目前还没有关于核仁靶向的双光子光动力治疗的报道。
将具有核仁靶向能力的光敏剂用于双光子的光动力治疗,能够最大程度地发挥光动力治疗的潜能。虽然最新的研究成果已经表明核仁是一个更加有效的肿瘤治疗新靶点[36-37],但是目前的绝大多数光敏剂并不能直接进入细胞核中,需要进行一定的修饰才能具有核仁靶向能力。最常用的修饰方法是在光敏剂表面修饰核定位信号[38-39],如用叶酸和姜黄素对碳纳米点(简称“碳点”)进行表面修饰,利用叶酸介导的内化机制使碳点具有核仁靶向能力;此外,使用具有核仁靶向能力的纳米颗粒作为光敏剂的载体,也能使光敏剂特异性靶向到核仁[40]。但是此类光敏剂普遍存在合成过程繁琐、修饰效率低、复合颗粒不稳定等问题,因此迫切需要找到一种合成简单、无需修饰的核仁靶向光敏剂,用于双光子光动力治疗。
本研究团队以柠檬酸和乙二胺为原材料,通过简单的微波加热法合成了一种具有核仁靶向能力的碳点,将其作为光敏剂,实现了高效的核仁靶向光动力治疗。此外,本研究团队表征了碳点的吸收光谱、荧光光谱和粒径,测定了碳点的双光子吸收截面、双光子激发荧光和双光子激发产生活性氧的能力,表征了碳点的细胞毒性和核仁靶向能力。随后,在740 nm波长飞秒激光作用下,研究了以碳点作为光敏剂的双光子光动力疗法对HeLa细胞的治疗效果。合成的碳点在实验中表现出了优异的性能和治疗效果,在双光子光动力治疗中具有一定的发展潜力。
2 实验材料与方法
2.1 实验材料
柠檬酸(CA)购于北京化学工业集团有限公司,乙二胺(EDA)购于福晨(天津)化学试剂有限公司,DMEM培养基和磷酸盐缓冲生理盐水(PBS, 1×) 购于HyClone公司,胎牛血清(FBS)购于ScienCell公司,碘化丙啶(PI)购于Sigma公司,钙黄绿素-AM(Calcein-AM)购于Biolegend公司,细胞计数试剂盒(CCK-8)购于Dojindo公司,SYTO RNASelect购于Invitrogen公司,HeLa(人类宫颈癌细胞系)购于ScienCell公司。
2.2 碳点的制备
称取1.26 g柠檬酸和1.65 mL乙二胺,将两者充分混匀后溶于30 mL超纯水中,再放入微波炉中用中高火加热,待观察到烧杯中的水分基本蒸干,液体变为深棕色黏稠状半固体时停止加热。将得到的产物溶于30 mL超纯水中,并用超声水浴锅使产物充分溶解,得到澄清的深棕色溶液。设定电热套温度为380 ℃,将重悬后的溶液放入50 mL烧杯中并在预热好的电热套上加热,待观察到溶液中的水分基本被蒸干,溶液变为棕褐色黏稠状半固体时开始计时,继续加热5 min进行碳化。
将得到的碳化产物冷却到室温并溶于20 mL超纯水中,取澄清溶液置于离心管中,以8000 r/min的速度离心10 min,取上清液再次进行离心,重复三次,去除溶液中的大颗粒碳化物。紧接着将上清液用孔径为200 nm的滤膜过滤,然后将得到的滤液转移到3000 Da(1 Da=1 u)超滤离心管中,以7500 r/min的速度离心10 min。将得到的滤液放入冻干机中冻干,得到深棕色粉末,此即为实验所需的小粒径碳点。
2.3 碳点物理性质的表征
在本研究中,使用紫外分光光度计(AuCy UV1901PC)测量碳点的吸收光谱,使用荧光光谱仪(HITACHI F2700)测量碳点的荧光光谱,使用动态光散射仪(Malvern ZETASIZER NANO)测量碳点的粒径。
2.4 CCK-8检验碳点的生物相容性
配制含有0,250,500,750 μg/mL碳点的培养基溶液。将HeLa细胞传入96孔板中,待细胞贴壁后将培养基替换成含相应质量浓度碳点的培养基,分别在孵育箱中培养24 h和48 h。孵育结束后用针筒吸出含碳点的培养基,并加入含10% CCK-8的培养基,放入孵育箱中反应1 h。用酶标仪(SpectraMax i3X)检测溶液在450 nm处的吸光度,以此表征材料的生物相容性。
2.5 碳点的双光子激发
750 nm飞秒激光通过物镜(16×)会聚后用于激发碳点溶液。用相机记录碳点的荧光发光情况,以衡量碳点的双光子吸收能力。
2.6 碳点核仁靶向能力检验
选用商用核仁染料SYTO RNASelect与碳点共染HeLa细胞,通过荧光显微成像验证碳点的核仁靶向能力。在实验过程中,通过设定不同的激发光波长和接收波段来区分碳点和SYTO RNASelect的荧光,其中:SYTO RNASelect的激发波长为488 nm,荧光接收范围为510~540 nm;碳点的激发波长为552 nm,荧光接收范围为560~590 nm。
2.7 碳点的活性氧产生能力
选用商用活性氧指示剂9,10-Anthracenediyl-bis(methylene)-dimalonic acid (ABDA)来表征碳点的活性氧产量,其检测原理是:活性氧可以消耗ABDA,使其紫外-可见光吸收光谱的特征吸收峰强度下降。通过测量ABDA特征吸收峰的变化来表征碳点的活性氧产生能力。
在本实验中,首先将ABDA粉末溶于二甲基亚砜(DMSO),配制成浓度为5 mmol/L的母液,然后用超纯水将碳点粉末配制成4 mg/mL的溶液。将1967.5 μL超纯水、20 μL ABDA母液以及12.5 μL 碳点溶液均匀混合,得到待测溶液。使用功率密度为100 mW/cm2的白光激发待测溶液,并在照射不同时间后,使用紫外-可见光分光光度计测量混合溶液的吸收光谱。
2.8 HeLa细胞的光动力治疗
用分析天平称取4 mg碳点,将称量好的碳点加入1 mL超纯水中配制成4 mg/mL碳点水溶液;用旋涡仪使碳点完全溶解,得到澄清的深棕色水溶液;用DMEM培养基稀释碳点水溶液至500 μg/mL,避光存放备用。
待共聚焦小皿中的HeLa细胞贴壁后,将培养基更换为含碳点的培养基,然后放在孵育箱中孵育3 h (37 ℃,5% CO2),用于细胞光动力治疗研究。使用飞秒激光(740 nm,28 mW)对细胞进行不同治疗时间的光动力治疗。治疗结束后,将共聚焦小皿放回孵育箱中孵育4 h,之后采用荧光成像方法评估细胞双光子光动力疗法的效果。
2.9 Calcein-AM/PI活死细胞染色
同时取PI和Calcein-AM各4 μL,用培养基配制浓度为2 μmol/L的染液。Calcein-AM可以穿过细胞膜进入细胞内,活细胞中的酯酶会脱去它的AM基团,产生Calcein,即钙黄绿素。钙黄绿素可以发出很强的绿色荧光,因此,活细胞在共聚焦显微镜下呈现绿色。PI不能穿过活细胞的细胞膜,但能通过死细胞受损的细胞膜进入胞内,并嵌入到细胞的DNA中。PI与DNA嵌合后会发出红色荧光,使死细胞在共聚焦显微镜下呈现红色。因此,选用PI和Calcein-AM共染细胞分别标定死细胞和活细胞,进而评估双光子光动力疗法的效果。
双光子光动力治疗4 h后,将PI/ Calcein-AM的混合染液与治疗后的HeLa细胞共孵育30 min;之后使用PBS清洗细胞两次,去除残留的PI和Calcein-AM,加入2 mL普通培养基,用于细胞成像。其中:Calcein-AM通道(活细胞通道)的激发波长为488 nm,荧光接收范围为510~540 nm;PI通道(死细胞通道)的激发波长为552 nm,荧光接收范围为560~590 nm。
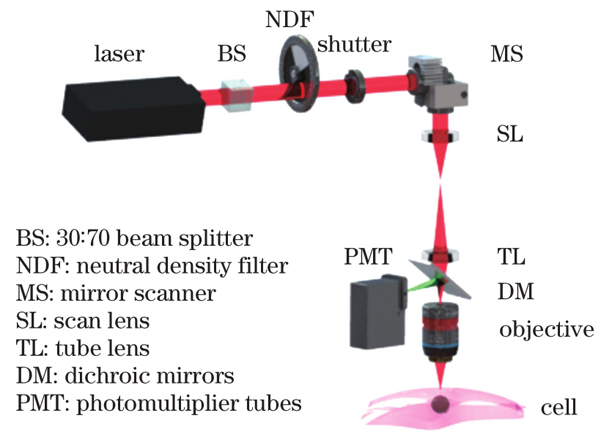
2.10 光动力治疗系统
如
3 分析与讨论
3.1 碳点的合成及表征
碳点是一种新兴的纳米材料,相比于量子点,碳点具有更好的生物安全性和快速代谢特性,已被广泛应用于生物医学光学成像和治疗中[41]。本研究团队选用柠檬酸和乙二胺作为原材料,通过微波加热法合成碳点。通过动态光散射(DLS)方法测得合成碳点的粒径为0.649 nm±0.124 nm,如
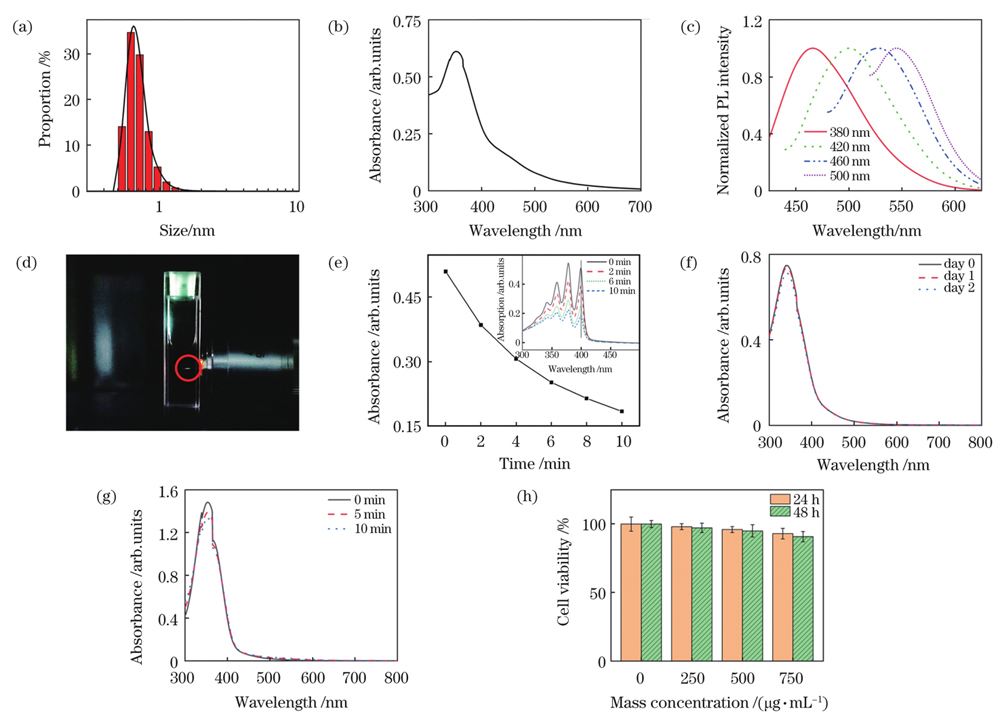
图 2. 碳点的性能表征。(a)粒径;(b)吸收光谱;(c)荧光光谱;(d)双光子激发荧光;(e)ABDA特征吸收峰(λ=399 nm)强度随光照时间的变化曲线,插图是碳点和ABDA混合溶液在不同时间光照下的紫外-可见光吸收光谱;(f)在PBS中放置不同时间后碳点的吸收光谱;(g)不同光照时间下碳点的吸收光谱;(h)生物相容性评估
Fig. 2. Characterization of C-dots. (a) Particle size; (b) absorption spectrum; (c) fluorescence spectra; (d) two-photon excited fluorescence; (e) variation curve of characteristic absorption peak (λ=399 nm) of ABDA under light irradiation with different light irradiation time, where the inset shows UV-Vis spectra of ABDA and C-dots under light irradiation for different time; (f) absorption spectra of C-dots in PBS for different time; (g) absorption spectra of C-dots under light irradiation for different time; (h) assessment of biocompatibility of C-dots
3.2 碳点的核仁靶向能力研究
为了研究碳点在细胞内的定位情况,将碳点(500 μg/mL)与HeLa细胞共孵育3 h后进行荧光成像。如
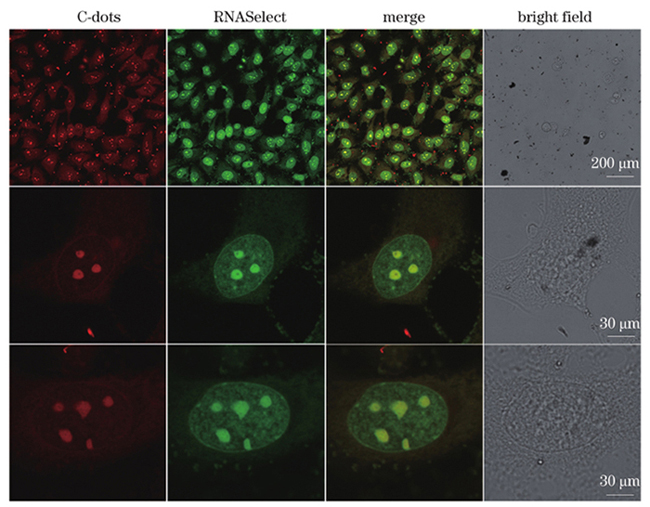
图 3. 碳点与RNASelect共染HeLa细胞的荧光图像、叠加图像及明场图像,其中碳点通道的激发波长λex=552 nm,发射波长λem=560~590 nm,RNASelect通道的激发波长λex=488 nm,发射波长λem=510~540 nm,RNASelect的浓度为10 μmol/L, 碳点的质量浓度为500 μg/mL
Fig. 3. Fluorescence images, merged images, and bright field images of HeLa cells stained with both RNASelect and C-dots, where excitation and emission wavelengths of C-dots channel are λex=552 nm and λem=560-590 nm, excitation and emission wavelengths of RNASelect channel are λex=488 nm and λem=510-540 nm, the concentration of RNASelect is 10 μmol/L, and the mass concentration of C-dots is 500 μg/mL
3.3 细胞的光动力治疗
为了分析碳点的细胞光动力治疗效率,将HeLa细胞与碳点(500 μg/mL)共孵育3 h,之后利用飞秒激光(740 nm,28 mW)对碳点标记的HeLa细胞进行光照。进行不同时间的光照后,选用Calcein-AM 和 PI对治疗后的HeLa细胞染色,并通过荧光成像方法评估治疗效果。如
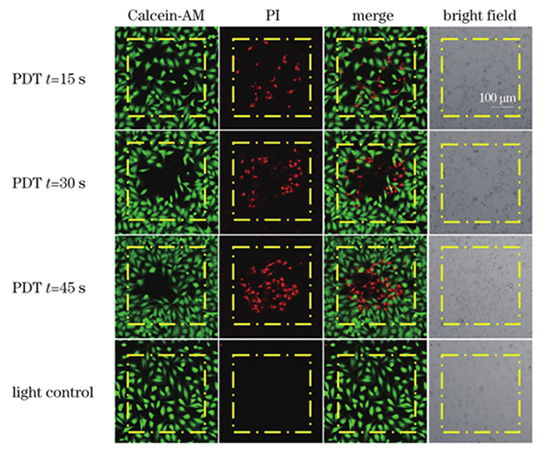
图 4. 核仁靶向双光子光动力疗法引起细胞坏死。孵育/未孵育碳点的HeLa细胞分别受到不同时间的双光子扫描,其中Calcein 通道的激发波长λex=488 nm,发射波长λem=505~525 nm,PI通道的激发波长λex=532 nm,发射波长λem=605~625 nm,黄色方块表示扫描区域
Fig. 4. Necrosis development in response to nucleolus-targeted two-photon PDT. HeLa cells incubated with/without C-dots were irradiated by two-photon scans with different time, where excitation and emission wavelengths of Calcein channel are λex=488 nm and λem=505-525 nm, excitation and emission wavelengths of propidium iodide (PI) channel are λex=532 nm and λem=605-625 nm, and scanned areas are indicated by yellow squares
表 1. 双光子光动力治疗中的光剂量
Table 1. Light dose for two-photon photodynamic therapy
|
4 结论
本研究团队设计并合成了具有核仁靶向和活性氧产生能力的新型碳点。核仁是一个更加有效的肿瘤治疗新靶点,本研究团队合成的碳点具有靶向核仁能力,可以在癌细胞核仁处产生活性氧,从而在低剂量的光照和光敏剂条件下,实现有效的肿瘤细胞双光子光动力治疗。合成的碳点具有良好的生物相容性和快速代谢等优势,并且双光子激发可以获得更大的穿透深度。因此,基于碳点的核仁靶向双光子光动力疗法在未来的转化医学研究中具有巨大潜力。
[1] Carelle N, Piotto E, Bellanger A, et al. Changing patient perceptions of the side effects of cancer chemotherapy[J]. Cancer, 2002, 95(1): 155-163.
[2] Browning R J, Reardon P J T, Parhizkar M, et al. Drug delivery strategies for platinum-based chemotherapy[J]. ACS Nano, 2017, 11(9): 8560-8578.
[3] Bruheim K, Guren M G, Skovlund E, et al. Late side effects and quality of life after radiotherapy for rectal cancer[J]. International Journal of Radiation Oncology Biology Physics, 2010, 76(4): 1005-1011.
[4] Chang-Claude J, Popanda O, Tan X L, et al. Association between polymorphisms in the DNA repair genes, XRCC1, APE1, and XPD and acute side effects of radiotherapy in breast cancer patients[J]. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research, 2005, 11(13): 4802-4809.
[5] Dolmans D E J G J, Fukumura D, Jain R K. Photodynamic therapy for cancer[J]. Nature Reviews Cancer, 2003, 3(5): 380-387.
[6] Castano A P, Mroz P, Hamblin M R. Photodynamic therapy and anti-tumour immunity[J]. Nature Reviews Cancer, 2006, 6(7): 535-545.
[7] 黄燕霞, 许皓, 栾萍, 等. β-淀粉样蛋白斑块的无标记成像及光动力降解[J]. 中国激光, 2020, 47(2): 0207029.
[8] MacDonald I J, Dougherty T J. Basic principles of photodynamic therapy[J]. Journal of Porphyrins and Phthalocyanines, 2001, 5(2): 105-129.
[9] Alfadda A A, Sallam R M. Reactive oxygen species in health and disease[J]. Journal of Biomedicine & Biotechnology, 2012, 2012: 936486.
[10] Railkar R, Agarwal P K. Photodynamic therapy in the treatment of bladder cancer: past challenges and current innovations[J]. European Urology Focus, 2018, 4(4): 509-511.
[11] Usuda J, Kato H, Okunaka T, et al. Photodynamic therapy (PDT) for lung cancers[J]. Journal of Thoracic Oncology, 2006, 1(5): 489-493.
[12] Agostinis P, Berg K, Cengel K A, et al. Photodynamic therapy of cancer: an update[J]. CA: a Cancer Journal for Clinicians, 2011, 61(4): 250-281.
[13] de FreitasL F, HamblinM R. Antimicrobial photoinactivation with functionalized fullerenes[M]//Grumezescu A M. Nanobiomaterials in antimicrobial therapy. Amsterdam: Elsevier, 2016: 1-27.
[14] Skovsen E, Snyder J W, Lambert J D C, et al. Lifetime and diffusion of singlet oxygen in a cell[J]. The Journal of Physical Chemistry B, 2005, 109(18): 8570-8573.
[15] Lin L Y, Pang W, Jiang X Y, et al. Light amplified oxidative stress in tumor microenvironment by carbonized hemin nanoparticles for boosting photodynamic anticancer therapy[J]. Light: Science & Applications, 2022, 11(47): 1-16.
[16] Zhou Z J, Song J B, Nie L M, et al. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy[J]. Chemical Society Reviews, 2016, 45(23): 6597-6626.
[17] 谢丽娜, 高诚宜, 汪琪, 等. 基于切伦科夫辐射的光动力疗法用于肿瘤治疗的研究进展[J]. 激光与光电子学进展, 2020, 57(19): 190002.
[18] 李阳, 王秀翃, 李艳艳, 等. 金属有机框架载药平台光动力消融乳腺癌细胞[J]. 激光与光电子学进展, 2021, 58(14): 1417002.
[19] Wu W, Mao D, Hu F, et al. A highly efficient and photostable photosensitizer with near-infrared aggregation-induced emission for image-guided photodynamic anticancer therapy[J]. Advanced Materials, 2017, 29(33): 1700548.
[20] Zhu H J, Li J C, Qi X Y, et al. Oxygenic hybrid semiconducting nanoparticles for enhanced photodynamic therapy[J]. Nano Letters, 2018, 18(1): 586-594.
[21] Ge J, Lan M, Zhou B, et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation[J]. Nature Communications, 2014, 5: 4596.
[22] Lü W, Zhang Z, Zhang K Y, et al. A mitochondria-targeted photosensitizer showing improved photodynamic therapy effects under hypoxia[J]. Angewandte Chemie (International Ed. in English), 2016, 55(34): 9947-9951.
[23] Zhang D Y, Zheng Y, Zhang H, et al. Ruthenium complex-modified carbon nanodots for lysosome-targeted one- and two-photon imaging and photodynamic therapy[J]. Nanoscale, 2017, 9(47): 18966-18976.
[24] Li W, Yang J, Luo L, et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death[J]. Nature Communications, 2019, 10: 3349.
[25] Tan W Y, Zhang Q X, Wang J Q, et al. Enzymatic assemblies of thiophosphopeptides instantly target Golgi apparatus and selectively kill cancer cells[J]. Angewandte Chemie (International Ed. in English), 2021, 60(23): 12796-12801.
[26] Pang W, Jiang P F, Ding S H, et al. Nucleolus-targeted photodynamic anticancer therapy using renal-clearable carbon dots[J]. Advanced Healthcare Materials, 2020, 9(16): e2000607.
[27] DeRosa M C, Crutchley R J. Photosensitized singlet oxygen and its applications[J]. Coordination Chemistry Reviews, 2002, 233/234: 351-371.
[28] Gu B B, Yong K T, Liu B. Strategies to overcome the limitations of AIEgens in biomedical applications[J]. Small Methods, 2018, 2(9): 1700392.
[29] 林立, 李步洪. 发光二极管在光动力疗法中的应用进展[J]. 激光与光电子学进展, 2020, 57(15): 150001.
[30] Bhawalkar J D, Kumar N D, Zhao C F, et al. Two-photon photodynamic therapy[J]. Journal of Clinical Laser Medicine & Surgery, 1997, 15(5): 201-204.
[31] Gu B, Wu W, Xu G, et al. Precise two-photon photodynamic therapy using an efficient photosensitizer with aggregation-induced emission characteristics[J]. Advanced Materials, 2017, 29(28): 1701076.
[32] Huang H Y, Yu B L, Zhang P Y, et al. Highly charged ruthenium(II) polypyridyl complexes as lysosome-localized photosensitizers for two-photon photodynamic therapy[J]. Angewandte Chemie (International Ed. in English), 2015, 54(47): 14049-14052.
[33] Collins H A, Khurana M, Moriyama E H, et al. Blood-vessel closure using photosensitizers engineered for two-photon excitation[J]. Nature Photonics, 2008, 2(7): 420-424.
[34] Gary-Bobo M, Mir Y, Rouxel C, et al. Mannose-functionalized mesoporous silica nanoparticles for efficient two-photon photodynamic therapy of solid tumors[J]. Angewandte Chemie (International Ed. in English), 2011, 50(48): 11425-11429.
[35] Zou Q L, Zhao H Y, Zhao Y X, et al. Effective two-photon excited photodynamic therapy of xenograft tumors sensitized by water-soluble bis(arylidene)cycloalkanone photosensitizers[J]. Journal of Medicinal Chemistry, 2015, 58(20): 7949-7958.
[36] Mijatovic T, de Nève N, Gailly P, et al. Nucleolus and c-Myc: potential targets of cardenolide-mediated antitumor activity[J]. Molecular Cancer Therapeutics, 2008, 7(5): 1285-1296.
[37] Hua X W, Bao Y W, Wu F G. Fluorescent carbon quantum dots with intrinsic nucleolus-targeting capability for nucleolus imaging and enhanced cytosolic and nuclear drug delivery[J]. ACS Applied Materials & Interfaces, 2018, 10(13): 10664-10677.
[38] Tian X H, Zhu Y Z, Zhang M Z, et al. Localization matters: a nuclear targeting two-photon absorption iridium complex in photodynamic therapy[J]. Chemical Communications, 2017, 53(23): 3303-3306.
[39] Nasrin A, Hassan M, Gomes V G. Two-photon active nucleus-targeting carbon dots: enhanced ROS generation and photodynamic therapy for oral cancer[J]. Nanoscale, 2020, 12(40): 20598-20603.
[40] Pan L M, Liu J N, Shi J L. Cancer cell nucleus-targeting nanocomposites for advanced tumor therapeutics[J]. Chemical Society Reviews, 2018, 47(18): 6930-6946.
[41] Panwar N, Soehartono A M, Chan K K, et al. Nanocarbons for biology and medicine: sensing, imaging, and drug delivery[J]. Chemical Reviews, 2019, 119(16): 9559-9656.
[42] Tong G S, Wang J X, Wang R B, et al. Amorphous carbon dots with high two-photon fluorescence for cellular imaging passivated by hyperbranched poly(amino amine)[J]. Journal of Materials Chemistry B, 2015, 3(4): 700-706.
[43] Han R C, Zhao M, Wang Z W, et al. Super-efficient in vivo two-photon photodynamic therapy with a gold nanocluster as a type I photosensitizer[J]. ACS Nano, 2020, 14(8): 9532-9544.
[44] He X J, Bo S T, Gao M, et al. Stereotactic photodynamic therapy using a two-photon AIE photosensitizer[J]. Small, 2019, 15(50): e1905080.
[45] Wang S W, Chen H, Liu J, et al. NIR-II light activated photosensitizer with aggregation-induced emission for precise and efficient two-photon photodynamic cancer cell ablation[J]. Advanced Functional Materials, 2020, 30(30): 2002546.
[46] Lesani P, Mohamad Hadi A H, Lu Z, et al. Design principles and biological applications of red-emissive two-photon carbon dots[J]. Communications Materials, 2021, 2: 108.
Article Outline
雷曼, 逄雯, 石擘, 王晨, 王丹, 魏勋斌, 顾波波. 基于核仁靶向碳点的双光子光动力疗法研究[J]. 中国激光, 2022, 49(15): 1507104. Man Lei, Wen Pang, Bo Shi, Chen Wang, Dan Wang, Xunbin Wei, Bobo Gu. Two-Photon Photodynamic Therapy Using Nucleolus-Targeted Carbon Dots[J]. Chinese Journal of Lasers, 2022, 49(15): 1507104.