光致超声技术及其应用  下载: 513次
下载: 513次
Biomedical ultrasound imaging has been widely used as an imaging technology based on ultrasound signals for viewing the internal structure of the body and finding the source of diseases. In recent decades, owing to the development of ultrasonic transducers, ultrasound imaging has made significant progress in obtaining important diagnostic information using rapid and noninvasive methods. Traditional transducers are excited by electricity and take advantage of the piezoelectric effect to achieve a transformation between electricity and ultrasound. However, considering the demanding requirements of application environments, the primary restriction is the limited bandwidth of traditional transducers.
Laser-generated ultrasound, a novel technology based on photoacoustic effect, is excited by a laser instead of electricity. Ultrasound pulses are generated by the absorption of a short-pulse laser, thus leading to elastic thermal expansion caused by the transient temperature increase. In this process, the time-varying laser acts as the only excitation source. The upper limits of the energy and frequency of the ultrasound are restricted by the laser. Compared with piezoelectric transducers, the ultrasound generated by laser-generated ultrasound transducers has the characteristics of high frequency and large bandwidth, which are necessary for sensing and imaging.
With the breakthrough of laser-generated ultrasound transducers in the structural simplification and excitation of large-bandwidth ultrasound, laser-generated ultrasound technology has been gradually applied in various fields where traditional piezoelectric ultrasound methods cannot be applied, essentially providing a novel idea for high-precision and high-resolution biomedical applications.
The amplitude of ultrasound produced by laser-generated ultrasound technology is related to various characteristics, such as laser energy, transducer absorbance, thermal expansion coefficient, and shape. Moderately high-energy laser, highly absorbing nano-scale light absorbers, and expanders with high thermal expansion coefficients positively affect the ultrasound amplitude generated by laser-generated ultrasound transducers. Meanwhile, the ultrasound frequency domain generated by photoconductive ultrasound technology is related to parameters such as the excitation light pulse width, transducer material, and transducer thickness (Figs. 2?4). For example, under test conditions in which the imaging depth is small but the imaging resolution is very high, an ultra-narrow pulse width laser with a nanoscale metal layer can be used as an optical ultrasound transducer (Table 1 and Fig. 2). If the test environment has high requirements for imaging depth and imaging speed but low requirements for imaging resolution, a common nanosecond transducer is suitable. If the test environment has high requirements for imaging depth and speed and low requirements for resolution imaging, a common nanosecond-pulsed laser with the carbon-based polymer material is suitable as a solution for ultrasound.
Moreover, the less complicated structure of the laser-generated ultrasound transducer promises a large amplitude of the ultrasound at the focal point, with a self-focusing effect when using a concave transducer (Figs. 1 and 5). Furthermore, the ultrasound generated by a laser-generated ultrasound transducer has a high frequency and large bandwidth, thereby contributing to a smaller sound field at the focal point (Fig. 6).
This study summarizes the mechanism of action, transducer system, performance characterization, and application areas of phototransduction ultrasound technology, as well as the applications of concave transducers in neural stimulation, ultrasonic cavitation, and ultrasound imaging, and describes the advantages and disadvantages of piezoelectric-based and photoacoustic effect-based transducers by comparing them with conventional ultrasound transducers. With the continuous development of theoretical systems of laser-generated ultrasound and precision processing technology, the advancement of laser-generated ultrasound technology has led to new opportunities for the development of biomedical ultrasound.
1 引言
超声波被定义为频率大于20 kHz的声能[1-3],由于其具有穿透能力强、低成本、安全性好、便利性高等优点,被广泛应用于生物医学等领域[4-6]。生物医学超声是一种利用超声波信号作为信息载体的成像技术,应用范围广泛,例如查看身体内部结构、寻找疾病来源和孕检[7-9]。目前,得益于复杂的信号处理技术和成像系统的集成及超声换能器的发展和完善[10-13],超声成像技术能以快速、无创的方式获取患者重要的诊断信息。
超声换能器作为超声系统中最重要的部件,决定了超声系统的主要性能和参数。传统超声是基于压电晶体利用逆压电效应和晶体共振,将电能转换为超声波(机械能)[14]。同时,超声换能器的材料类型、形状、大小、频率等参数可以根据实际应用进行调整,目前不同类型的换能器被应用于各个领域,如心脏病学、产科、妇科、泌尿科、骨科和眼科[15-16]。经过几十年的发展,超声换能器在理论和实践方面都取得了长足的进步[17-20],然而传统的超声波发射器受到现代应用的挑战。在应用环境的苛刻要求下,超声换能器的一些缺点也被暴露出来,比如:有限的带宽限制了成像分辨率[21-26];压电材料与生物组织或者水之间存在巨大的声阻抗差异,导致需要增加一层甚至多层匹配层,这增加了制造难度[27-29]。
利用光声效应产生超声是基于传统压电效应产生超声波的一种替代方法,其光-声转换过程对应于传统压电换能器的电-声转换过程[30],对应的器件称为光致超声换能器。光致超声换能器吸收短脉冲光,瞬态温度升高,产生弹性热膨胀,从而产生超声脉冲。光致超声换能器可以实现几百MHz甚至GHz的超声,且所产生的超声具有高频率和宽带宽的特性,这满足了高分辨率成像的需要。同时,光致超声换能器的小型化结构有利于探测器的微型化,可用于体积受限部位的神经刺激或者影像学研究。此外,光致超声换能器不易受电磁的干扰,易与磁共振成像系统兼容,这些优点使光致超声换能器成为了临床以及科研中精准、可靠的成像工具。
本文回顾了光致超声技术的基本特点,介绍了光致超声技术的工作机制、基本架构、声场分布等,并概述了光致超声技术的应用,特别是在生物医学领域中的应用。
2 光致超声换能器
2.1 光致超声换能器的机制
光声效应是指材料吸收光能,发生热膨胀,产生超声的过程[31-34]。传统的光声成像也是利用光声效应产生超声,但是其成像目标一般为组织或者固体样品,其中样品直接暴露在激光中[35-46]。相比之下,光致超声换能器是利用光声效应在成像目标外部产生超声波[47-48]。另外,光致超声换能器可以与光学检测超声器件配合使用,从而组成全光学超声成像器件,应用于生物医学[49-54]。
光致超声换能器的主要机制是吸收激光后的热膨胀[55-57]。这主要涉及到两个能量转换过程:光能到热能,热能到机械能(超声)[58-59]。光声效应有两个限定条件:热约束条件和应力约束条件。热约束条件是指激励激光器的脉冲持续时间应短于热弛豫时间,即在光子与物质发生相互作用期间,传导到物质外的热量可以忽略不计。压力约束条件是指,激励激光器的脉冲持续时间应短于压力弛豫时间,即在光子与物质发生相互作用期间,物质的热膨胀变化可以忽略不计。这意味着激光的脉冲宽度应同时小于光致超声换能器的热弛豫时间和压力弛豫时间,只有满足这两个必要条件,换能器产生的超声声压才能最大化[60]。
光致超声换能器在利用脉冲激光或者调制激光基于光声效应产生超声波(机械波)的过程中,时变激光作为光致超声过程中唯一的激励源,其能量决定了产生的超声能量的上限,同时时变激光的频域决定了超声频域的上限[61-63]。
对于非黏性介质,基于光声效应的超声波产生和传播由光声方程[64-65]给出:
式中:
2.2 光致超声换能器的材料体系
为了最大限度地提高光声转换效率,人们对光致超声材料进行了探索。光致超声换能器材料由最初的单层光吸收薄膜发展为目前的复合材料。复合材料通常由光吸收体和热膨胀体组成,光吸收体高效地吸收激光能量并将其转换为热量,热膨胀体将热高效地转化为机械波(超声)。
对于光吸收体,金属和碳化合物是常见的材料,包括金[66-68]、铬[69-70]、锗[71]、钙钛矿(MAPbI3)[72]、石墨烯[73]、炭黑(CB)[74-75]、蜡烛烟灰(CS)[76-78]、碳纳米管(CNTs)[79-86]及碳纳米纤维(CNFs)[61]。虽然金属材料基于共振效应在某些波长下具有高的光吸收率,但碳材料具有宽带吸收并易与各种激光系统兼容的优点,得到了广泛应用。对于热膨胀材料,聚二甲基硅氧烷(PDMS)由于其高的热膨胀性和透明度而备受关注[87]。目前,光致超声换能器的光吸收体主要分为金属和碳材料两类。
英国诺丁汉大学的La Cavera等[88]利用脉宽为100 fs的脉冲激光激励溅射涂覆在光纤端面的15 nm厚的金层,得到了GHz级别频率的声波,实现了2.5 μm的光学横向分辨率和纳米级的轴向分辨率,展示了光致超声换能器在超精细影像方面的能力。德国慕尼黑工业大学的Seeger等[66]在170 μm厚的玻璃盖玻片上沉积了两层金属层,分别是4 nm厚的钛层(作为黏合层)和250 nm厚的金层,用于高频、大带宽超声的激发。在该研究中,厚度为4 nm的钛层在532 nm的垂直入射光下透射了约13%的激光功率,厚度为250 nm的金层在532 nm的垂直入射光下吸收了约29%的激光功率,这显示了金属材料对激光的低吸收效率,相关信息也被其他文献[32]报道。以上是以金属作为光吸收体的典型的光致超声换能器,从
表 1. 不同类型的光致超声换能器
Table 1. Summary of different types of laser-generated ultrasound transducers
|
因此,具有高吸收率的基于碳材料的光致超声换能器引起了研究人员的关注。美国密歇根大学的Buma等[74]利用载玻片上沉积的0.15 mm厚的铬膜产生了超声信号,并与炭黑-PDMS发声体系进行了比较,结果显示,炭黑-PDMS的光致超声能量转换效率比铬膜高24 dB。在该研究中,PDMS的体积线性热膨胀系数为3×10-6 μm2/℃,几乎是水的5倍,比许多金属的热膨胀系数高出一个数量级,且辐射声脉冲的频谱与激光脉冲的光谱相当。美国密歇根大学的Won Baac等[61]在熔融二氧化硅基底上生长了多壁碳纳米管,然后旋涂PDMS,制备了CNT-PDMS复合材料,结果显示,CNT-PDMS复合膜的信号振幅比铬材料的信号振幅高14 dB。美国北卡罗莱纳州立大学的Chang等[89]将载玻片放置在蜡烛火焰上方,将蜡烛灰沉积在载玻片上,然后将PDMS旋涂到蜡烛灰上,制备了蜡烛灰-PDMS的光致超声换能器,得到了9.69×10-3的超高光致超声转换效率。上述研究结果表明,碳基的光致超声换能器相比于基于金属的换能器具有更高的能量转换效率,故更多类型、结构的碳基光致超声换能器被开发出来。
北京航空航天大学的Wang等[54]利用多壁碳纳米管(MWCNT)和聚甲基丙烯酸甲酯(PMMA)制备了MWCNT-PMMA薄膜,并在凸形模具的配合下,制备了微型的凹形光致超声换能器,用于产生高频的聚焦超声。英国伦敦大学学院的Noimark等[80]利用油胺官能化的芘、二甲苯和丙酮等试剂,基于多壁碳纳米管和PDMS制成了MWCNT-PDMS凝胶,然后通过提拉法在光纤端面制备了MWCNT-PDMS光致超声换能器,用于对猪主动脉进行成像。韩国成均馆大学的Faraz等[90]采用火焰合成工艺,利用石蜡蜡烛在室温下获得了蜡烛烟灰碳纳米颗粒(CSNPs),然后将沉积的CSNPs转移到玻璃瓶中,利用己烷和CSNPs混合液体制作出CSNPs-PDMS材料,结果表明,CSNPs-PDMS发射机的中心频率为2.44~13.34 MHz,6 dB带宽范围为5.80~13.62 MHz。
光致超声换能器正在快速发展中,目前虽尚未建立完整的理论体系,但已经取得了一定的共识,比如高吸光度的纳米尺度光吸收体和热膨胀系数高的膨胀体在光致超声换能器中的应用是重要的。高吸光度的材料用于高效吸收激光能量,从而高效地将激光能量转变为热量。纳米尺度的光吸收体允许热量快速转移到周围的介质中。随着热扩散时间的减少,这种快速、高效的热传递可以引导膨胀体更快地发生热膨胀,从而产生高幅值、高带宽超声。
2.3 光致超声换能器的构建以及结构
传统典型的超声换能器通常由晶体层、声学匹配层和背衬层组成[41,91]。晶体层通常由压电材料制成,材料主要是压电陶瓷[主要成分是锆钛酸铅(PZT)],通过施加高频交流电压,压电陶瓷可以通过逆压电效应(将电能转换为机械能)发生高频振动[92]。然而,压电陶瓷元件和生物组织或者水环境之间巨大的声阻抗差异阻碍了超声波能量在两种介质之间的有效传递[93-94]。声学匹配层用于促进超声能量的传输,每个匹配层在换能器中心频率处的厚度为四分之一波长的整数倍,通常需要配置一层、两层甚至是三层的声学匹配层[95-99]。同时,为了消除背向超声干扰,减小超声脉冲宽度,需要配置吸声背衬,用于吸收从压电元件向后传播的超声波[100-102]。如果后波在背衬块底部反射并返回压电元件,则会在超声图像中引起噪声,因此,背衬块应具有高衰减[103-104]的特性。如果背衬块的声阻抗过高,压电元件产生的声能将在背衬层大量损失,传输到人体的超声波会大幅度减少[105-106]。除此之外,在压电陶瓷、声学匹配层和阻尼层之间需要使用超声耦合剂,而压电陶瓷与声学匹配层的厚度只有几十微米甚至几微米,这无疑增大了探头的制作难度和技术门槛[107]。
与压电换能器不同的是,光致超声换能器结构较为简单,实现方式多样。相比于传统压电超声换能器繁琐复杂的制备过程[108],光致超声换能器的制备较为简单,只需要将光吸收体和膨胀体制成复合材料即可。传统超声换能器必备的三层(至少三层)结构体系为匹配层-晶体层-背衬层,而且三层结构需要利用声阻抗匹配的胶水精密连接,而光致超声换能器一般只有一层结构。当然,无论是压电换能器还是光致超声换能器,两者都需要外界激励才能产生超声,压电换能器需要负压电脉冲激励,光致超声换能器需要脉冲激光激励。值得注意的是,目前商用脉冲激光的使用成本高于商用电脉冲的使用成本。在传统超声换能器中,由于压电层与水(或者组织)之间的声阻抗差异巨大[常用压电层材料PZT5的声阻抗为34 Mrayl,水的声阻抗为1.48 Mrayl,其中1 Mrayl=106 kg/(m2·s)],声学匹配层的存在是必要的。而光致超声换能器材料(一般为PDMS)的声阻抗约为1.26 Mrayl,与水非常接近,所以在光致超声换能器中无须配置声学匹配层。同时,光致超声换能器受时变激光作用产生热膨胀,当激光停止作用时,没有动力使换能器继续形变,且换能器不会持续振荡,所以同样无须配置背衬。光致超声换能器典型的获得方法如下。
2012年,美国密歇根大学的Baac等[109]通过化学气相沉积方法,在球透镜(二氧化硅)衬底上生长了多壁碳纳米管,并在CNT膜上沉积了20 nm厚的金层,然后将聚合物聚二甲基硅氧烷旋涂在Au/CNT层上,制备了聚焦光致超声换能器。2013年,英国伦敦大学学院的Mattiat[91]将光纤置于PDMS和多壁碳纳米管的复合溶液中,通过提拉法在光纤端面制备了基于MWCNT-PDMS的光致超声换能器。2018年,美国北卡罗莱纳州立大学的Chang等[89]将载玻片置于石蜡蜡烛火焰上方2 cm处,使炭黑积累在载玻片上,然后通过旋涂的方式将PDMS融入炭黑中,制备了基于炭黑-PDMS的光致超声换能器,并探究了蜡烛烟灰的结构和厚度对光声转导效率的影响。2020年,德国慕尼黑工业大学的Seeger等[66]使用电子束辅助沉积方法,在170 μm厚的玻璃盖玻片上沉积了两层金属(4 nm厚的钛层和250 nm厚的金层),将厚度为254 nm的金属作为光致超声换能器。2022年,美国波士顿大学的Li等[75]通过将蜡烛烟灰纳米颗粒嵌入到弯曲的PDMS膜中,得到了聚焦的光致超声换能器。
![压电超声换能器的典型结构以及不同种类的光致超声换能器。(a)压电超声换能器的典型结构[96];(b)基于光纤的光致超声换能器,比例尺50 μm[80];(c)全向的光致超声换能器结构示意图[110];(d)全向的光致超声换能器用于产生全向超声[110]](/richHtml/zgjg/2023/50/21/2107105/img_01.jpg)
图 1. 压电超声换能器的典型结构以及不同种类的光致超声换能器。(a)压电超声换能器的典型结构[96];(b)基于光纤的光致超声换能器,比例尺50 μm[80];(c)全向的光致超声换能器结构示意图[110];(d)全向的光致超声换能器用于产生全向超声[110]
Fig. 1. Typical structures of piezoelectric ultrasonic transducers and different kinds of laser-generated ultrasound transducers. (a) Typical structure of piezoelectric ultrasonic transducer[96]; (b) laser-generated ultrasound transducer based on fiber with scale bar of 50 μm[80]; (c) structural diagram of omnidirectional laser-generated ultrasound transducer[110]; (d) omni-directional laser-generated ultrasound transducers used to generate omni-directional ultrasound[110]
光致超声技术的运行机制明确、实现方式较为简单,对光致换能器的构建没有严格的要求。同时,光致超声换能器的材料获取方便且经济,对实验室的基础设备要求低,可以根据实际应用环境进行灵活调整,极大扩展了其应用范围。
3 光致超声换能器的性能
在光致超声换能器中,光致超声材料与器件结构决定了声压、光-声转换效率、光致超声频率和声场。
3.1 光致超声声压的影响因素以及转换效率
声压是光致超声换能器应用过程中较为重要的指标,如果超声换能器产生的超声无法被常用的超声检测设备检测到,该换能器的应用将受到极大的限制。
光致超声换能器利用脉冲光或调制光通过光声效应将激光能量转换为超声(机械波),产生的超声振幅(P0)[64-65]可以表示为
式中:
光致超声换能器的光-声转换效率也是值得探讨的一个参数。相比于基于压电材料的超声换能器的电-声转换效率(20%~50%),光致超声换能器的光-声转换效率偏低。目前已经公布的数据显示,Cr材料具有0.042%的转换效率[71]、蜡烛烟尘颗粒/PDMS材料具有0.441%的转换效率[78],碳纳米管/PDMS复合材料具有0.959%的转换效率[89],碳纳米管/金/PDMS材料具有2.74%的转换效率[18],钙钛矿-PDMS具有2.97%的转换效率[72]。光致超声换能器的能量转换过程分为光致热和热致超声两个主要步骤,虽然光致热的能量转换效率较高,但受限于热膨胀体的热膨胀系数,热致超声过程的转换效率较低,总转换效率较低[59]。同时,光致超声的频率范围极宽,甚至可以到达GHz的水平,而目前市售的水听器的检测带宽有限,只能检测到某一范围内的超声波,导致检测到的转换效率较低。
相比于传统超声换能器,凹形光致超声换能器的应用非常普遍。通过凹形换能器的自聚焦效应,超声换能器在焦点处的声压比非聚焦换能器强。而且凹形光致超声换能器的聚焦效果比压电换能器优,这是因为光致超声换能器产生的超声具有高频、大带宽性能,有利于在焦点处的产生更小的声场
超声换能器的数值孔径(NA)定义为换能器横向直径与凹形换能器曲率半径的比值[16,75,109]。对于传统的小型超声换能器,在制备过程中由于压电晶体可能会产生裂纹,因此单晶压电材料不易达到高NA[59,75]。然而,微型化的光致超声换能器可以容易地获得高达0.95的NA,接近理论极限值[75]。这么高的NA不仅为聚焦换能器提供了高横向分辨率,而且在焦点处提供了高聚焦增益。增益(G)由焦点处的压力与球面上的压力之比定义[109]:
式中:
类似于压电换能器的有效工作电压,光致超声换能器也存在有效激光能量,或者称为安全激光强度,该参数限定了作用于光致超声换能器的激光阈值。相关研究[80]显示,随着作用于光致超声换能器的激光能量的增加,换能器产生的声压增加,但是在激光能量达到阈值(产生的超声峰峰值达到4.5 MPa左右的阈值压力)后,超声功率显著降低,且光致超声换能器没有出现明显损伤,在较低的激光通量下仍能正常工作。Guo团队研究发现,当激光脉冲宽度为6 ns时,CNT-PDMS复合膜的损伤阈值为280~300 mJ/cm2,比相同配置下PDMS涂覆的金纳米结构和金属薄膜高7~8倍;光致超声换能器材料的热损伤阈值是决定最大可用激光能量的重要因素[109]。
3.2 光致超声频域
光致超声换能器利用脉冲激光或者调制激光通过光声效应产生超声波。为了获得所需的光致超声脉冲,应首先选择合适脉冲宽度的激励光。在光致超声过程中,时变激光作为光致超声过程中唯一的激励源,对应的频域决定了超声频域的上限[59]。
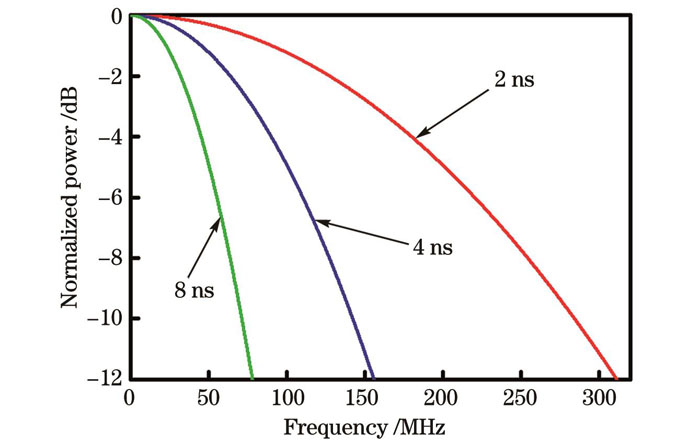
图 2. 不同脉宽的激光对应的频域分布
Fig. 2. Frequency domain distributions of laser with different pulse widths
同时,光声脉冲的带宽不仅由激光脉冲宽度决定,还由空间吸收轮廓决定。当使用非常薄的光致超声发声膜时,光声脉冲的带宽可以达到由激光脉冲宽度设置的上限。而当光吸收体具有大厚度时,光声脉冲的带宽小于上限。
研究认为,在光致超声过程中,热量导致介质发生热膨胀,热源可以被划分为数个薄片,每个薄片都会发出具有相同时域分布的声波,这些声波的叠加产生最终的超声,即光致超声换能器发出的超声波正比于时间加热函数与空间光吸收函数的卷积,如
![光致超声换能器厚度对光致超声脉冲的影响[59]。(a)激光作用于不同厚度光致超声换能器的示意图;(b)用卷积积分确定不同厚度光致超声换能器的光致超声脉冲示意图](/richHtml/zgjg/2023/50/21/2107105/img_03.jpg)
图 3. 光致超声换能器厚度对光致超声脉冲的影响[59]。(a)激光作用于不同厚度光致超声换能器的示意图;(b)用卷积积分确定不同厚度光致超声换能器的光致超声脉冲示意图
Fig. 3. Influence of thickness of laser-generated ultrasound transducer on laser-generated ultrasound pulse[59]. (a) Schematics of laser acting on laser-generated ultrasound transducers with different thicknesses; (b) schematics of determining laser-generated ultrasound pulses of laser-generated ultrasound transducers with different thickness by convolution integral
在不同文献中,由于超声产生系统的参数(激光脉宽、材料体系、材料厚度、激光性能等)和超声检测系统的参数(检测类型、检测角度等)具有差异性,因此不同文献的数据难以对比。因此,我们选择相同实验条件下的数据进行对比,展示系统参数的单个变量对超声频域的影响。
2013年,德国慕尼黑工业大学的Omar等[115]在琼脂凝胶中掺杂了直径为10 μm的黑色聚苯乙烯微球(浓度为2×102 /mm3),制作了光致超声模具,使用脉冲宽度为1.8 ns和8 ns的纳秒激光分别照射该模具,并使用中心频率为78 MHz、带宽为25~125 MHz的球面聚焦超声探测器检测该光致超声模具发出的超声波,结果显示,不同激光脉宽下的超声谱存在很大的差异,脉冲宽度为1.8 ns和8 ns的激光产生的超声峰值频率响应分别为~40 MHz和130 MHz,如
![不同激光脉宽和材料厚度下的时域以及频域信息。(a)光致超声系统原理图[115];(b)10 μm直径黑色微球在不同激光脉冲时长激励下的频域分布[115];(c)光致超声的时域分布[115];(d)光致超声方法得到的10 μm直径微球的重建图像[115];(e)低激光能量输入下不同沉积时间(10、30、120 s)样品的声压和声谱[89]](/richHtml/zgjg/2023/50/21/2107105/img_04.jpg)
图 4. 不同激光脉宽和材料厚度下的时域以及频域信息。(a)光致超声系统原理图[115];(b)10 μm直径黑色微球在不同激光脉冲时长激励下的频域分布[115];(c)光致超声的时域分布[115];(d)光致超声方法得到的10 μm直径微球的重建图像[115];(e)低激光能量输入下不同沉积时间(10、30、120 s)样品的声压和声谱[89]
Fig. 4. Time domain and frequency domain information under different laser pulse widths and material thicknesses. (a) Schematic of laser-generated ultrasound system[115]; (b) frequency domain distribution of 10 μm diameter black microsphere under excitation with different laser pulse durations[115]; (c) time domain distribution of laser-generated ultrasound[115]; (d) reconstructed image of 10 μm diameter microsphere obtained by laser-generated ultrasound method[115]; (e) sound pressures and spectra of samples with different deposition time (10, 30, 120 s) obtained under low laser energy input[89]
2018年,美国北卡罗莱纳州立大学的Chang等[89]使用脉冲激光作用厚度分别为2.15、6.48、25.8 μm的CS-PDMS光致超声换能器,产生的超声主频分别为~17、~16、~9 MHz,带宽分别为22.8、22.6、19 MHz,结果表明,随着光致超声换能器厚度的增加,产生的超声主频和带宽均降低,如
由此可见,光致超声频谱不仅与激发光脉冲宽度有关,还与换能器材料、换能器厚度等参数有关。研究人员可以根据研究目标,选择合适的光致超声换能器参数。比如,在成像深度较浅但对成像分辨率有极高要求的测试条件中,可以利用纳米尺度的金属层作为光致超声换能器,并使用超窄脉宽激光激励该金属层;如果测试环境对成像深度和成像速度有较高要求但对成像分辨率的要求较低,选择常用的纳秒级脉冲激光配合碳基-聚合物材料作为光致超声的合适方案。
3.3 声场分布
光致超声换能器与传统基于压电材料的超声换能器具有不同的声场分布[116]。对于任何形状和大小的换能器,其有效的振源均可看成许多小面积的声源,每个小面积的声源都可看成一个单一的换能器[117]。每个换能器相应的超声场形状都可以由换能器衍射图确定。
在基于压电材料的超声换能器中,压电晶体经极化处理后,振动方向为其极化方向,产生的超声场指向性较好[118]。而相较于纳米厚度的光致超声换能器微声源,基于压电材料的超声换能器的微声源面积较大,产生的超声声场指向性更好。
在光致超声换能器中,纳米尺度的光吸收体将热量传输给周围的介质(热膨胀体)。热膨胀体是全向扩散的,最终得到的声场较为发散。华中科技大学的Tang团队利用COMSOL Multiphysics软件模拟了非聚焦光致超声换能器的声场分布,在不考虑超声衰减的情况下,换能器直径为5 mm,声波长度为50 μm,其自然焦距约为12.5 mm;在焦距以内,超声波束宽度基本保持不变,接近换能器的尺寸(5 mm);在焦距之外,随着离焦距离的增加,超声波束会变得越来越宽,如
![光致超声换能器的声场分布。(a)仿真得到的平面超声产生的声场分布[72];(b)实验测得的平面超声产生的声场分布[119];(c)仿真聚焦型超声产生的声场分布[54];(d)聚焦型超声换能器产生的示意图[75];(e)全向超声的声场分布[124]](/richHtml/zgjg/2023/50/21/2107105/img_05.jpg)
图 5. 光致超声换能器的声场分布。(a)仿真得到的平面超声产生的声场分布[72];(b)实验测得的平面超声产生的声场分布[119];(c)仿真聚焦型超声产生的声场分布[54];(d)聚焦型超声换能器产生的示意图[75];(e)全向超声的声场分布[124]
Fig. 5. Distribution of sound field of laser-generated ultrasound transducer. (a) Simulated sound field distribution generated by planar ultrasound[72]; (b) experimental sound field distribution generated by planar ultrasound[119]; (c) simulated sound field distribution generated by focusing ultrasound[54]; (d) schematic of focusing ultrasonic transducer generation[75]; (e) sound field distribution of omnidirectional ultrasound[124]
光致超声换能器具有较简单的结构,极易加工为凹形,从而产生聚焦的声场。美国密歇根大学的Baac等[109]通过在凹透镜上涂覆CNT-Au-PDMS复合层,制备了聚焦的超声换能器,产生了峰值为50 MPa的超声波,完成了对模型肾结石的切割。美国北卡罗莱纳州立大学的Kim等[120]将炭黑/聚二甲基硅氧烷混合物旋涂在平凹玻璃透镜(直径为12.5 mm,曲率半径为12.4 mm)上,制备了聚焦的光致超声换能器,用于体外超声溶栓。体外测试结果表明,微泡介导的光致聚焦超声(LGFU)治疗可实现2 mg/min左右的溶解速率,这表明LGFU换能器可实现精确的高速率声溶解。北京航空航天大学的王璞团队使用K-wave软件仿真了聚焦半径为3 mm的光致超声换能器的声场分布,其中光致超声换能器的激发面积为0.5 mm×0.5 mm,产生的超声中心频率为40 MHz、带宽为200%[121-123]。仿真结果显示,该聚焦光致超声换能器产生了聚焦的超声,超声焦点距离换能器2.5 mm,同时距离换能器3 mm处的声场直径为200 μm,距离换能器6 mm处的声场直径为400 μm,如
在某些特定的应用中,光致超声换能器须产生更为发散的超声分布,比如全向的超声分布。美国波士顿大学的Lan等[124]为了对病变部位进行高精度定位,利用光致超声换能器产生了全向超声。他们在光纤尖端开发了一种两层纳米复合材料结构,以产生全方向的声波,其强度足以深入组织,如
3.4 光致超声换能器的缺点与不足
光致超声换能器作为一种新型的超声产生器件,在带宽超声激发、抗电磁干扰等方面表现出明显的优势。然而,光致超声换能器受超声产生条件和过程及换能器架构等因素的影响,在激发效率、成本、适应性等方面尚存不足。
如3.1节所述,相比于传统基于压电材料的超声换能器,光致超声换能器的能量转换效率较低,最高能量转换效率约为2.97%[72]。造成这一结果的原因主要是光能转换为热量、热量引起热膨胀等一系列过程的效率低,特别是热量引起热膨胀的过程。在热量引起热膨胀的过程中,复合材料的光声转换效率受到热膨胀体(主要为PDMS)热膨胀系数的限制,高温造成PDMS性能退化。报道显示,PDMS的Grüneisen系数随着激光强度的增加而降低[59],这进一步影响了能量转换效率。因此,为了提高光声转换效率,探索具有更高热膨胀系数和材料稳健性的其他聚合物具有重要意义。当然,光致超声换能器的低能量转换效率可以通过配置凹形的换能器结构进行部分补偿,但是这无疑增加了加工难度。
与基于压电材料的超声换能器类似,光致超声换能器也需要外部能量的激励才能产生超声波。压电换能器利用超声波脉冲发生器产生的脉冲负压作为激励源,光致超声换能器利用脉冲激光器产生的脉冲激光作为激励源,然而目前商用超声波脉冲发生器的价格低于商用脉冲激光器的价格。虽然商用超声波发生器受益于压电换能器较广的使用范围和较低的价格,但是大规模应用的成本仍较高,故基于压电材料的超声换能器在系统成本控制方面更具优势。
同时,一个重要的区别是,压电超声换能器是双向的,而光致超声换能器是单向的。压电超声换能器既能激发超声也能响应超声,而光致超声换能器只能激发超声,其必须搭配其他器件才能实现超声的响应。
综上所述,相比于压电换能器,光致超声换能器具有压电换能器无法比拟的优势,但同时也存在缺点,光致超声换能器适用于难以应用压电超声方法的高标准要求环境。
4 光致超声换能器的应用
4.1 脑部神经刺激
为了了解大脑的功能及其功能障碍导致疾病的机理,需要采用超高精度调节神经元活动的方式。毫米精度的大脑刺激模式通常会激活多个功能区域并引起误导性的反应[125],有限的空间精度阻碍了单个大脑区域的激活,使得小鼠运动皮层的绘制变得困难[126]。对于大鼠海马特定细胞层的精确靶向刺激,需要优于0.2 mm的空间精度[127]。然而,传统的电刺激工具、经颅光遗传学和经颅聚焦超声都无法安全实现高精度的神经刺激[128-129]。
美国波士顿大学的Li等[75]报道了光学聚焦超声(OFUS)换能器,用于超高精度的非侵入性脑刺激,如
![体外OFUS经颅刺激[75]。(a)经颅体外刺激示意图;(b)经颅刺激前后神经元的代表性图像,比例尺为50 μm;(c)经颅OFUS刺激的平均钙反应轨迹;(d)单周期直接刺激和经颅刺激的阈值压力统计](/richHtml/zgjg/2023/50/21/2107105/img_06.jpg)
图 6. 体外OFUS经颅刺激[75]。(a)经颅体外刺激示意图;(b)经颅刺激前后神经元的代表性图像,比例尺为50 μm;(c)经颅OFUS刺激的平均钙反应轨迹;(d)单周期直接刺激和经颅刺激的阈值压力统计
Fig. 6. Transcranial stimulation in vitro by OFUS[75]. (a) Schematic of transcranial stimulation in vitro; (b) representative images of neurons before and after transcranial stimulation with scale bar of 50 μm; (c) averaged calcium response trace of transcranial OFUS stimulation; (d) statistics of threshold pressure of direct and transcranial stimulation in single cycle
此外,该工作为了将光子有效转换为声波的最佳吸收体,制备并测试了基于四种不同光声材料的光致超声换能器,包括热收缩膜(HSM)、与PDMS混合的碳纳米管CNT-PDMS、与PDMS混合的碳纳米颗粒及与PDMS分层的蜡烛烟灰。通过测量焦点处的压力,比较它们的光声转换效率。CS-PDMS的光致超声材料是最有效的,在激光功率密度为0.62 mJ/cm2的激光输入下,在超声焦点处产生了~48 MPa的声压。
4.2 血管内全光学超声成像
心血管疾病(CVD)已成为人类的主要死因[130],临床主要通过识别易损性斑块来评估心血管疾病。易损性斑块的独特特征是薄的纤维帽和大的坏死核心[131],所以对易损性斑块进行识别需要影像技术具有高分辨率和大探测深度,以提供整体的、高分辨率的组织学信息。血管内成像技术是对冠状动脉血管壁的形态学进行可视化分析,评估心肌梗死风险,以引导冠脉临床心内介入治疗,商用的血管内介入影像技术主要有血管内超声(IVUS)和光学相干断层扫描(OCT)[132]。血管内超声成像优势是成像的范围(>6 mm)很大,但成像分辨率较差;OCT具有高分辨性能,然而受到组织高散射特性的影响,成像深度被限制为1~2 mm。所以目前的血管技术面临的重大问题是:高分辨率与大成像深度不能兼得,难以提供整体的、高分辨的血管内壁形态学信息[133]。
北京航空航天大学的Wang等[54]报道了一种血管内超声成像系统,该系统利用皮秒激光脉冲泵浦碳复合材料,进行超声激发,并利用π-相移光纤布拉格光栅(FBG)进行超声检测,如
![超宽带AO-IVUS的成像[54]。(a)AO-IVUS血管壁成像示意图;(b)AO-IVUS导管超声响应功率谱;(c)AO-IVUS获取的二维截面图像;(d)AO-IVUS旋转-回拉扫描动脉壁三维超声数据](/richHtml/zgjg/2023/50/21/2107105/img_07.jpg)
图 7. 超宽带AO-IVUS的成像[54]。(a)AO-IVUS血管壁成像示意图;(b)AO-IVUS导管超声响应功率谱;(c)AO-IVUS获取的二维截面图像;(d)AO-IVUS旋转-回拉扫描动脉壁三维超声数据
Fig. 7. Imaging by ultra-wide bandwidth AO-IVUS[54]. (a) Imaging diagram of vessel wall by AO-IVUS; (b) power spectrum of AO-IVUS catheter ultrasound response; (c) 2D cross-sectional image acquired by AO-IVUS; (d) 3D ultrasound data of arterial wall by rotation-pullback scan with AO-IVUS
关于光致超声换能器的参数表征和应用类型,更多研究关注的是光致超声换能器的声压强度以及凹形换能器产生的小焦点,往往忽略了光致超声换能器的超大带宽对超声成像分辨率的巨大影响。对于超声成像系统,图像的空间分辨率定义为可以区分的两个相邻特征之间的最小距离[134]。空间分辨率越高,可以区分的距离越小。由于二维横截面超声图像同时显示血管壁的深度和感兴趣部分的宽度,因此空间分辨率进一步细分为轴向分辨率和横向分辨率。轴向分辨率(Raxial)也称为深度或纵向分辨率,其特征是沿超声波束轴线区分紧密相邻特征的能力,可以表示为Raxial=c/(2fcBW)=λ/(2BW),其中c是声速,fc是超声换能器的中心频率,BW是超声换能器的-6 dB分数带宽,λ是波长[135-136]。中心频率为70~200 MHz的超声换能器的典型轴向分辨率范围为20~50 μm[137]。对于传统的超声换能器,由于带宽无法提升,只能通过提高主频来提高轴向分辨率[138]。但是,随着主频的提高,组织对超声的衰减和散射作用增加,系统成像深度降低,传统的超声系统就需要在图像分辨率和穿透深度之间权衡[139]。而光致超声换能器能够产生高频、大带宽的超声,可极大降低轴向分辨率,从而提高成像分辨率。
4.3 高精度靶向细胞去除
高振幅聚焦超声可以在聚焦体积内诱导冲击、声空化和热沉积,在液体和组织中提供局部扰动[140-143]。这种机械和热扰动常被用于向细胞和组织提供靶向冲击,用于生物医学治疗,如跨膜药物递送[144-146]、大脑中的神经活动调节[147-148]和血栓溶解[149]。然而,传统超声换能器产生的焦点尺寸较大,无论是高频还是低频的传统超声换能器,都无法在外科手术中高精度消融病变组织[150]。
美国密歇根大学的Baac等[109]利用激光产生聚焦超声,该超声可以在直径仅为6 mm的单元件透镜中产生高频(>15 MHz)的50 MPa的光致超声声压。聚焦超声是通过独特设计的光声换能器产生的,该换能器由碳纳米管-聚合物复合材料制成,在凹面上直接实现声聚焦,可用于非侵入性超声治疗。该研究团队在凹透镜上制备碳纳米管和弹性体聚合物的纳米复合膜,并利用具有高光学吸收效率和高热传递效率的CNT作为有效的光声源。该聚焦光致超声换能器可以在横向宽度为75 mm、轴向宽度为400 mm的紧密焦斑上产生峰值为50 MPa的光声压力,该压力振幅非常高,可以在焦点区域内诱导显著的冲击效应和非热脉冲空化。光声透镜可以用于固体材料的微尺度超声破碎(
![LGFU对固体材料的微观破碎[109]。(a)模型肾结石(比例尺为4 mm);(b)通过单个LGFU脉冲在聚合物膜上产生单个微孔(比例尺为20 μm);(c)(d)聚合物涂覆玻璃基板上的碎裂过程的高速显微镜图像](/richHtml/zgjg/2023/50/21/2107105/img_08.jpg)
图 8. LGFU对固体材料的微观破碎[109]。(a)模型肾结石(比例尺为4 mm);(b)通过单个LGFU脉冲在聚合物膜上产生单个微孔(比例尺为20 μm);(c)(d)聚合物涂覆玻璃基板上的碎裂过程的高速显微镜图像
Fig. 8. Micro-scale fragmentation of solid materials by LGFU[109]. (a) Model of kidney stone with scale bar of 4 mm; (b) single micro-hole on polymer film produced by single LGFU pulse with scale bar of 20 μm; (c)(d) high-speed microscopic images of fragmentation process on polymer-coated glass substrate
![LGFU对目标细胞的去除(比例尺为520 mm)[95]。(a)超声暴露前培养的卵巢癌细胞(SKOV3);(b)LGFU暴露后选择性地去除单个细胞;(c)当LGFU点移动到黑点区域时细胞互连被切断](/richHtml/zgjg/2023/50/21/2107105/img_09.jpg)
图 9. LGFU对目标细胞的去除(比例尺为520 mm)[95]。(a)超声暴露前培养的卵巢癌细胞(SKOV3);(b)LGFU暴露后选择性地去除单个细胞;(c)当LGFU点移动到黑点区域时细胞互连被切断
Fig. 9. Targeted cell removal by LGFU with scale bar of 520 mm[95]. (a) Cultured ovarian cancer cells (SKOV3) before ultrasound exposure; (b) selective removal of single cell after LGFU exposure; (c) cellular interconnection is severed when LGFU spot is moved to black dot region
4.4 病变部位定位
肿块切除术也称为保乳手术,是早期乳腺癌症的标准手术治疗方法,有利于保乳、降低发病率和快速康复[151-153]。然而,在肿块切除术中,当病变部位很小且无法触及时,准确定位肿瘤是一项挑战[154]。这种困难可能导致肿瘤切除不完全或手术时间延长,从而导致高二次手术率(约25%)和手术成本的增加[155-156]。
美国波士顿大学的Lan等[124]报道了一种具有增强现实(AR)功能的光纤光声引导(FOG)技术,用于亚毫米肿瘤定位和低干扰的直观手术引导,如
![使用 FOG和AR系统定位肿瘤并切除肿瘤[124]。(a)原理示意图;(b)小车上紧凑综合系统的照片;(c)人体样本乳腺FOG尖端的可视化](/richHtml/zgjg/2023/50/21/2107105/img_10.jpg)
图 10. 使用 FOG和AR系统定位肿瘤并切除肿瘤[124]。(a)原理示意图;(b)小车上紧凑综合系统的照片;(c)人体样本乳腺FOG尖端的可视化
Fig. 10. Locating and removal of tumor using FOG and AR system[124]. (a) Principle diagram; (b) photograph of compact integrated system on cart; (c) visualization of FOG tip in breast of human sample
![用于肿瘤定位的光纤光声导管,精度为亚毫米[110]。(a)光纤光声波导、氧化锌纳米颗粒和环氧树脂的照片;(b)不同角度下FOG尖端产生的光声信号的信噪比;(c)向前方向上距离FOG尖端8 cm处记录的代表性光声信号波形;(d)检测器响应归一化后的代表性光声信号波形的频谱;(e)通过不同厚度的鸡胸肉组织后光声信号的信噪比](/richHtml/zgjg/2023/50/21/2107105/img_11.jpg)
图 11. 用于肿瘤定位的光纤光声导管,精度为亚毫米[110]。(a)光纤光声波导、氧化锌纳米颗粒和环氧树脂的照片;(b)不同角度下FOG尖端产生的光声信号的信噪比;(c)向前方向上距离FOG尖端8 cm处记录的代表性光声信号波形;(d)检测器响应归一化后的代表性光声信号波形的频谱;(e)通过不同厚度的鸡胸肉组织后光声信号的信噪比
Fig. 11. Fiber optoacoustic guide for tumor localization with sub-millimeter accuracy[111]. (a) Photographs of fiber optoacoustic guide, zinc oxide nanoparticles, and epoxy; (b) signal-to-noise ratio of generated optoacoustic signal from FOG tip at different angles; (c) representative optoacoustic signal waveform recorded at 8 cm away from FOG tip in forward direction; (d) frequency spectrum of representative optoacoustic signal waveform after normalization of detector response; (e) signal-to-noise ratio of generated optoacoustic signal after passing chicken breast tissues with different thicknesses
4.5 无喷嘴液体微喷射
液滴形成过程中的液体喷射在自然界中被广泛观察到。液体喷射的基本机制随着驱动力的变化而变化,从而导致不同的喷射特性。从无针药物注射[157-158]到高分辨率喷墨打印[159],微型液体射流的应用得到了广泛关注。在各种喷射方法中,最简单的一种方法是基于液体的不可压缩性,利用压电驱动膜[160]的变形或脉冲激光器[161]、电放电器[162]或电加热器[163]的热膨胀引起的液体容器的体积变化,将少量液体推出喷嘴。但由于喷嘴的存在,喷射过程容易受到喷嘴堵塞的影响,特别是当喷射液体包含薄片、颗粒或表面粘附生物分子时。
美国密歇根大学的Lee等[164]提出了一种基于光致超声原理产生超声波,实现液体无喷嘴喷射的方法,如
![空气-水界面处的阴影图像(比例尺为100 μm)[164]。(a)水微射流;(b)气泡](/richHtml/zgjg/2023/50/21/2107105/img_12.jpg)
图 12. 空气-水界面处的阴影图像(比例尺为100 μm)[164]。(a)水微射流;(b)气泡
Fig. 12. Shadowgraph images at air-water interface with scale bar of 100 μm[164]. (a) Water micro-jets; (b) bubbles
5 结束语
光致超声换能器技术作为压电技术的补充,在带宽超声等方面展现出优异的性能,然而在使用成本和能量转换效率方面却存在天然不足。目前光致超声发展尚处于初期,需要解决的问题较多。首先是光致超声体系尚未建立,对于光致超声的主频、带宽、转换效率等核心参数,目前尚未建立起类似于压电超声换能器的知识结构体系,这是未来需要解决的一个难题。其次,较高的使用成本限制了光致超声换能器的应用范围,光致超声技术适用于对超声成像有更高要求、体积受限、受复杂电磁干扰等场景,比如血管内超声成像。值得一提的是,在血管内成像时,将光致超声换能器与OCT、拉曼成像等完全基于光学的影像技术结合,不再配置电学器件,从而减小了血管内窥探头的直径并降低了成本。最后,面对光致超声换能器相对较低的能量转换效率,自聚焦的换能器结构利用凹面的聚焦增益,在焦点处产生较大的压力振幅,有助于缓解低能量转换效率带来的不足。同时,更换热膨胀体以提高转换效率或者改进工艺以提高换能器的空化阈值,这些措施将对更多的应用产生积极的影响。
光致超声换能器在结构简化和激发大带宽超声等方面的突破,使得光致超声技术逐渐应用于无法应用传统压电超声的领域,为高精度、高分辨的生物医学应用提供了新的思路。总结了光致超声技术的作用机制、换能器体系、性能表征和应用领域,详细介绍了凹形换能器在神经刺激、超声空化、超声成像等方面的应用,并且通过与传统超声换能器的对比,描述了基于压电效应和基于光声效应的换能器的优势与缺点。随着信号处理和精密加工技术的不断发展,光致超声换能器的研制与设计以及生物医学超声的发展也迎来了新的机会。高集成化、高效和简易的光致超声换能器是未来的研究方向。另外,通过与其他新型技术(比如光学检测超声技术)的融合,光致超声技术有望得到新的突破和应用。
[1] Leighton T G. What is ultrasound?[J]. Progress in Biophysics and Molecular Biology, 2007, 93(1/2/3): 3-83.
[2] Lu J Y, Zou H H, Greenleaf J F. Biomedical ultrasound beam forming[J]. Ultrasound in Medicine & Biology, 1994, 20(5): 403-428.
[3] Kolios M. Biomedical ultrasound imaging from 1 to 1000 MHz[J]. Canadian Acoustics, 2009, 37(3): 35-43.
[4] Zheng H R, Mukdadi O, Shandas R. Theoretical predictions of harmonic generation from submicron ultrasound contrast agents for nonlinear biomedical ultrasound imaging[J]. Physics in Medicine and Biology, 2006, 51(3): 557-573.
[5] Wang L, Lei P, Wen X, et al. Tapered fiber-based intravascular photoacoustic endoscopy for high-resolution and deep-penetration imaging of lipid-rich plaque[J]. Optics Express, 2019, 27(9): 12832-12840.
[6] Rousseau G, Gauthier B, Blouin A, et al. Non-contact biomedical photoacoustic and ultrasound imaging[J]. Journal of Biomedical Optics, 2012, 17(6): 061217.
[7] Carovac A, Smajlovic F, Junuzovic D. Application of ultrasound in medicine[J]. Acta Informatica Medica, 2011, 19(3): 168-171.
[8] Barnett S B, Ter Haar G R, Ziskin M C, et al. International recommendations and guidelines for the safe use of diagnostic ultrasound in medicine[J]. Ultrasound in Medicine & Biology, 2000, 26(3): 355-366.
[9] Foley J L, Vaezy S, Crum L A. Applications of high-intensity focused ultrasound in medicine: spotlight on neurological applications[J]. Applied Acoustics, 2007, 68(3): 245-259.
[10] Abdullah A, Shahini M, Pak A. An approach to design a high power piezoelectric ultrasonic transducer[J]. Journal of Electroceramics, 2009, 22(4): 369-382.
[11] Jung J, Lee W, Kang W, et al. Review of piezoelectric micromachined ultrasonic transducers and their applications[J]. Journal of Micromechanics and Microengineering, 2017, 27(11): 113001.
[12] Chen X Y, Lam K H, Chen R M, et al. Acoustic levitation and manipulation by a high-frequency focused ring ultrasonic transducer[J]. Applied Physics Letters, 2019, 114(5): 054103.
[13] Chen D D, Hou C X, Fei C L, et al. An optimization design strategy of 1-3 piezocomposite ultrasonic transducer for imaging applications[J]. Materials Today Communications, 2020, 24: 100991.
[14] Lee W, Roh Y. Ultrasonic transducers for medical diagnostic imaging[J]. Biomedical Engineering Letters, 2017, 7(2): 91-97.
[15] AzhariH. Basics of biomedical ultrasound for engineers[M]. Hoboken: John Wiley & Sons, Inc., 2010.
[16] CobboldR S C. Foundations of biomedical ultrasound[M]. Oxford: Oxford University Press, 2006.
[17] SuetensP. Fundamentals of medical imaging[M]. Cambridge: Cambridge University Press, 2017.
[18] Chen Z Y, Wu Y, Yang Y, et al. Multilayered carbon nanotube yarn based optoacoustic transducer with high energy conversion efficiency for ultrasound application[J]. Nano Energy, 2018, 46: 314-321.
[19] Wang X B, Yan F, Liu X F, et al. Enhanced drug delivery using sonoactivatable liposomes with membrane-embedded porphyrins[J]. Journal of Controlled Release, 2018, 286: 358-368.
[20] Zhu P C, Peng H M, Mao L L, et al. Piezoelectric single crystal ultrasonic transducer for endoscopic drug release in gastric mucosa[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2021, 68(4): 952-960.
[21] Ma T, Yu M Y, Li J W, et al. Multi-frequency intravascular ultrasound (IVUS) imaging[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2015, 62(1): 97-107.
[22] Yoon S, Kim M G, Williams J A, et al. Dual-element needle transducer for intravascular ultrasound imaging[J]. Journal of Medical Imaging, 2015, 2(2): 027001.
[23] Munding C E, Chérin E, Alves N, et al. 30/80 MHz bidirectional dual-frequency IVUS feasibility evaluated in vivo and for stent imaging[J]. Ultrasound in Medicine & Biology, 2020, 46(8): 2104-2112.
[24] Qiu W B, Chen Y, Wong C M, et al. A novel dual-frequency imaging method for intravascular ultrasound applications[J]. Ultrasonics, 2015, 57: 31-35.
[25] Su M, Zhang Z Q, Hong J H, et al. Cable-shared dual-frequency catheter for intravascular ultrasound[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2019, 66(5): 849-856.
[26] Munding C E, Chérin E, Jourard I, et al. Development of a 3 French dual-frequency intravascular ultrasound catheter[J]. Ultrasound in Medicine & Biology, 2018, 44(1): 251-266.
[27] Toda M, Thompson M. Detailed investigations of polymer/metal multilayer matching layer and backing absorber structures for wideband ultrasonic transducers[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2012, 59(2): 231-242.
[28] Nissen S E, Yock P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications[J]. Circulation, 2001, 103(4): 604-616.
[29] Opieliński K J, Gudra T. Influence of the thickness of multilayer matching systems on the transfer function of ultrasonic airborne transducer[J]. Ultrasonics, 2002, 40(1/2/3/4/5/6/7/8): 465-469.
[30] Li J P, Ma Y Q, Zhang T, et al. Recent advancements in ultrasound transducer: from material strategies to biomedical applications[J]. BME Frontiers, 2022, 2022: 9764501.
[31] McDonald F A, Wetsel G C. Generalized theory of the photoacoustic effect[J]. Journal of Applied Physics, 1978, 49(4): 2313-2322.
[32] Xu M H, Wang L V. Photoacoustic imaging in biomedicine[J]. Review of Scientific Instruments, 2006, 77(4): 041101.
[33] Wang L V. Prospects of photoacoustic tomography[J]. Medical Physics, 2008, 35(12): 5758-5767.
[34] Beard P. Biomedical photoacoustic imaging[J]. Interface Focus, 2011, 1(4): 602-631.
[35] Rosencwaig A, Gersho A. Theory of the photoacoustic effect with solids[J]. Journal of Applied Physics, 1976, 47(1): 64-69.
[36] Tam A C. Applications of photoacoustic sensing techniques[J]. Reviews of Modern Physics, 1986, 58(2): 381-431.
[37] Yao J J, Wang L V. Photoacoustic microscopy[J]. Laser & Photonics Reviews, 2013, 7(5): 758-778.
[38] Wen X E, Lei P, Huang S, et al. High-fluence relay-based disposable photoacoustic-ultrasonic endoscopy for in vivo anatomical imaging of gastrointestinal tract[J]. Photonics Research, 2022, 11(1): 55-64.
[39] Wang Z Y, Yang F, Ma H G, et al. Photoacoustic and ultrasound (PAUS) dermoscope with high sensitivity and penetration depth by using a bimorph transducer[J]. Journal of Biophotonics, 2020, 13(9): e202000145.
[40] 穆根, 张振辉, 石玉娇. 生物医学影像中的光声成像技术[J]. 中国激光, 2022, 49(20): 2007208.
[41] 王瑞荣, 崔丹丹, 石玉娇. 光敏感AgBr@PLGA纳米探针用于近红外二区肿瘤特异性光声成像[J]. 中国激光, 2022, 49(20): 2007204.
[42] 付武兵, 梁贻智, 仲晓轩, 等. 光纤光声血氧饱和度测量与功能成像[J]. 光学学报, 2022, 42(20): 2017001.
[43] 赵鹏艳, 陈重江. 多模态光声成像技术及其在眼科学中的应用进展[J]. 激光与光电子学进展, 2022, 59(6): 0617014.
[44] 李长辉. 用光奏响生命之歌:光声成像技术漫谈[J]. 激光与光电子学进展, 2022, 59(6): 0617005.
[45] 何勇, 廖唐云, 吴俊伟, 等. 基于透明超声换能器的光声显微镜设计[J]. 中国激光, 2022, 49(3): 0307001.
[46] Jin X, Wang X K, Xiong K D, et al. High-resolution and extended-depth-of-field photoacoustic endomicroscopy by scanning-domain synthesis of optical beams[J]. Optics Express, 2019, 27(14): 19369-19381.
[47] Kim J, Kim H, Chang W Y, et al. Candle-soot carbon nanoparticles in photoacoustics: advantages and challenges for laser ultrasound transmitters[J]. IEEE Nanotechnology Magazine, 2019, 13(3): 13-28.
[48] Noimark S, Colchester R J, Poduval R K, et al. Polydimethylsiloxane composites for optical ultrasound generation and multimodality imaging[J]. Advanced Functional Materials, 2018, 28(9): 1704919.
[49] Ansari R, Zhang E Z, Desjardins A E, et al. All-optical forward-viewing photoacoustic probe for high-resolution 3D endoscopy[J]. Light: Science & Applications, 2018, 7: 75.
[50] Alles E J, Noimark S, Maneas E, et al. Video-rate all-optical ultrasound imaging[J]. Biomedical Optics Express, 2018, 9(8): 3481-3494.
[51] Guggenheim J A, Li J, Allen T J, et al. Ultrasensitive plano-concave optical microresonators for ultrasound sensing[J]. Nature Photonics, 2017, 11(11): 714-719.
[52] Li G Y, Guo Z D, Chen S L. Miniature all-optical probe for large synthetic aperture photoacoustic-ultrasound imaging[J]. Optics Express, 2017, 25(21): 25023-25035.
[53] Hsieh B Y, Chen S L, Ling T, et al. All-optical scanhead for ultrasound and photoacoustic imaging-imaging mode switching by dichroic filtering[J]. Photoacoustics, 2014, 2(1): 39-46.
[54] Wang L, Zhao Y W, Zheng B, et al. Ultrawide-bandwidth high-resolution all-optical intravascular ultrasound using miniaturized photoacoustic transducer[J]. Science Advances, 2023, 9(23): eadg8600.
[55] Kim D, Ye M, Grigoropoulos C P. Pulsed laser-induced ablation of absorbing liquids and acoustic-transient generation[J]. Applied Physics A, 1998, 67(2): 169-181.
[56] Wang L V. Tutorial on photoacoustic microscopy and computed tomography[J]. IEEE Journal of Selected Topics in Quantum Electronics, 2008, 14(1): 171-179.
[57] Wu N, Tian Y, Zou X T, et al. High-efficiency optical ultrasound generation using one-pot synthesized polydimethylsiloxane-gold nanoparticle nanocomposite[J]. Journal of the Optical Society of America B, 2012, 29(8): 2016-2020.
[58] LeeT, BaacH W, OkJ G, et al. Polymer-nanomaterial composites for optoacoustic conversion[M]∥Li Q A. Functional organic and hybrid nanostructured materials. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA, 2018: 519-546.
[59] Lee T, Baac H W, Li Q C, et al. Efficient photoacoustic conversion in optical nanomaterials and composites[J]. Advanced Optical Materials, 2018, 6(24): 1800491.
[60] Paltauf G, Dyer P E. Photomechanical processes and effects in ablation[J]. Chemical Reviews, 2003, 103(2): 487-518.
[61] Won Baac H, Ok J G, Park H J, et al. Carbon nanotube composite optoacoustic transmitters for strong and high frequency ultrasound generation[J]. Applied Physics Letters, 2010, 97(23): 234104.
[62] Biagi E, Margheri F, Menichelli D. Efficient laser-ultrasound generation by using heavily absorbing films as targets[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2001, 48(6): 1669-1680.
[63] Hou Y, Kim J S, Ashkenazi S, et al. Broadband all-optical ultrasound transducers[J]. Applied Physics Letters, 2007, 91(7): 073507.
[64] WangL V, WuH I. Biomedical Optics: Principles and Imaging[M]. Hoboken: John Wiley & Sons, Inc., 2009.
[65] 石玉娇, 邢达. 纳米尺度下的光声效应及纳米探针光声转换机制研究[J]. 中国激光, 2018, 45(2): 0207026.
[66] Seeger M, Soliman D, Aguirre J, et al. Pushing the boundaries of optoacoustic microscopy by total impulse response characterization[J]. Nature Communications, 2020, 11: 2910.
[67] Hou Y, Kim J S, Ashkenazi S, et al. Optical generation of high frequency ultrasound using two-dimensional gold nanostructure[J]. Applied Physics Letters, 2006, 89(9): 093901.
[68] Zou X T, Wu N, Tian Y, et al. Broadband miniature fiber optic ultrasound generator[J]. Optics Express, 2014, 22(15): 18119-18127.
[69] Chen Y S, Zhao Y, Yoon S J, et al. Miniature gold nanorods for photoacoustic molecular imaging in the second near-infrared optical window[J]. Nature Nanotechnology, 2019, 14(5): 465-472.
[70] Kang S, Yoon Y, Kim J, et al. Thermoelastic response of thin metal films and their adjacent materials[J]. Applied Physics Letters, 2013, 102(2): 021908.
[71] Lee T, Guo L J. Highly efficient photoacoustic conversion by facilitated heat transfer in ultrathin metal film sandwiched by polymer layers[J]. Advanced Optical Materials, 2017, 5(2): 1600421.
[72] Du X Y, Li J P, Niu G D, et al. Lead halide perovskite for efficient optoacoustic conversion and application toward high-resolution ultrasound imaging[J]. Nature Communications, 2021, 12: 3348.
[73] Colchester R J, Alles E J, Desjardins A E. A directional fibre optic ultrasound transmitter based on a reduced graphene oxide and polydimethylsiloxane composite[J]. Applied Physics Letters, 2019, 114(11): 113505.
[74] Buma T, Spisar M, O’Donnell M. High-frequency ultrasound array element using thermoelastic expansion in an elastomeric film[J]. Applied Physics Letters, 2001, 79(4): 548-550.
[75] Li Y M, Jiang Y, Lan L, et al. Optically-generated focused ultrasound for noninvasive brain stimulation with ultrahigh precision[J]. Light: Science & Applications, 2022, 11: 321.
[76] Chang W Y, Zhang X A, Kim J, et al. Evaluation of photoacoustic transduction efficiency of candle soot nanocomposite transmitters[J]. IEEE Transactions on Nanotechnology, 2018, 17(5): 985-993.
[77] Zhou J, Jokerst J V. Miniature fiber-optic high-intensity focused ultrasound device using a candle soot nanoparticles-polydimethylsiloxane composites-coated photoacoustic lens: a review[J]. Photoacoustics, 2020, 20: 100211.
[78] Chang W Y, Huang W B, Kim J, et al. Candle soot nanoparticles-polydimethylsiloxane composites for laser ultrasound transducers[J]. Applied Physics Letters, 2015, 107(16): 161903.
[79] Silva A D, Henriques C A, Malva D V, et al. Photoacoustic generation of intense and broadband ultrasound pulses with functionalized carbon nanotubes[J]. Nanoscale, 2020, 12(40): 20831-20839.
[80] Noimark S, Colchester R J, Blackburn B J, et al. Carbon-nanotube-PDMS composite coatings on optical fibers for all-optical ultrasound imaging[J]. Advanced Functional Materials, 2016, 26(46): 8390-8396.
[81] Baac H W, Ok J G, Lee T, et al. Nano-structural characteristics of carbon nanotube-polymer composite films for high-amplitude optoacoustic generation[J]. Nanoscale, 2015, 7(34): 14460-14468.
[82] Li J P, Xu J B, Liu X L, et al. A novel CNTs array-PDMS composite with anisotropic thermal conductivity for optoacoustic transducer applications[J]. Composites Part B: Engineering, 2020, 196: 108073.
[83] Finlay M C, Mosse C A, Colchester R J, et al. Through-needle all-optical ultrasound imaging in vivo: a preclinical swine study[J]. Light: Science & Applications, 2017, 6(12): e17103.
[84] Oser P, Jehn J, Kaiser M, et al. Fiber-optic photoacoustic generator realized by inkjet-printing of CNT-PDMS composites on fiber end faces[J]. Macromolecular Materials and Engineering, 2021, 306(2): 2000563.
[85] Poduval R K, Noimark S, Colchester R J, et al. Optical fiber ultrasound transmitter with electrospun carbon nanotube-polymer composite[J]. Applied Physics Letters, 2017, 110(22): 223701.
[86] Huang W B, Chang W Y, Kim J, et al. A novel laser ultrasound transducer using candle soot carbon nanoparticles[J]. IEEE Transactions on Nanotechnology, 2016, 15(3): 395-401.
[87] Bodian S, Colchester R J, Macdonald T J, et al. CuInS2 quantum dot and polydimethylsiloxane nanocomposites for all-optical ultrasound and photoacoustic imaging[J]. Advanced Materials Interfaces, 2021, 8(20): 2100518.
[88] La Cavera S, Pérez-Cota F, Smith R J, et al. Phonon imaging in 3D with a fibre probe[J]. Light: Science & Applications, 2021, 10: 91.
[89] Chang W Y, Zhang X A, Kim J, et al. Evaluation of photoacoustic transduction efficiency of candle soot nanocomposite transmitters[J]. IEEE Transactions on Nanotechnology, 2018, 17(5): 985-993.
[90] Faraz M, Abbasi M A, Sang P, et al. Stretchable and robust candle-soot nanoparticle-polydimethylsiloxane composite films for laser-ultrasound transmitters[J]. Micromachines, 2020, 11(7): 631.
[91] MattiatO E. Ultrasonic transducer materials[M]. New York: Springer Science & Business Media, 2013.
[92] Zhou Q F, Cannata J, Shung K K. Design and modeling of inversion layer ultrasonic transducers using LiNbO3 single crystal[J]. Ultrasonics, 2006, 44: e607-e611.
[93] Zhou Q F, Lau S, Wu D W, et al. Piezoelectric films for high frequency ultrasonic transducers in biomedical applications[J]. Progress in Materials Science, 2011, 56(2): 139-174.
[94] Zhou Q F, Lam K H, Zheng H R, et al. Piezoelectric single crystal ultrasonic transducers for biomedical applications[J]. Progress in Materials Science, 2014, 66: 87-111.
[95] Callens D, Bruneel C, Assaad J. Matching ultrasonic transducer using two matching layers where one of them is glue[J]. NDT & E International, 2004, 37(8): 591-596.
[96] Ma X W, Cao W W. Single-crystal high-frequency intravascular ultrasound transducer with 40-μm axial resolution[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2020, 67(4): 810-816.
[97] Bian J C, Wang Y, Liu Z J, et al. Ultra-wideband underwater acoustic transducer with a gradient impedance matching layer[J]. Applied Acoustics, 2021, 175: 107789.
[98] Inoue T, Ohta M, Takahashi S. Design of ultrasonic transducers with multiple acoustic matching layers for medical application[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 1987, 34(1): 8-16.
[99] Hadimioglu B, La Comb L J, Jr, Wright D R, et al. High efficiency, multiple layer ZnO acoustic transducers at millimeter-wave frequencies[J]. Applied Physics Letters, 1987, 50(23): 1642-1644.
[100] Grewe M G, Gururaja T R, Shrout T R, et al. Acoustic properties of particle/polymer composites for ultrasonic transducer backing applications[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 1990, 37(6): 506-514.
[101] Nguyen N T, Lethiecq M, Karlsson B, et al. Highly attenuative rubber modified epoxy for ultrasonic transducer backing applications[J]. Ultrasonics, 1996, 34(6): 669-675.
[102] Hou C X, Fei C L, Li Z X, et al. Optimized backing layers design for high frequency broad bandwidth ultrasonic transducer[J]. IEEE Transactions on Biomedical Engineering, 2022, 69(1): 475-481.
[103] Toda M, Thompson M. Novel multi-layer polymer-metal structures for use in ultrasonic transducer impedance matching and backing absorber applications[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2010, 57(12): 2818-2827.
[104] Sayers C M, Tait C E. Ultrasonic properties of transducer backings[J]. Ultrasonics, 1984, 22(2): 57-60.
[105] Kuscer D, Bustillo J, Bakarič T, et al. Acoustic properties of porous lead zirconate titanate backing for ultrasonic transducers[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2020, 67(8): 1656-1666.
[106] El-Tantawy F, Sung Y K. A novel ultrasonic transducer backing from porous epoxy resin-titanium-silane coupling agent and plasticizer composites[J]. Materials Letters, 2004, 58(1/2): 154-158.
[107] Fei C L, Yang Y H, Guo F F, et al. PMN-PT single crystal ultrasonic transducer with half-concave geometric design for IVUS imaging[J]. IEEE Transactions on Bio-Medical Engineering, 2018, 65(9): 2087-2092.
[109] Baac H W, Ok J G, Maxwell A, et al. Carbon-nanotube optoacoustic lens for focused ultrasound generation and high-precision targeted therapy[J]. Scientific Reports, 2012, 2: 989.
[110] Menz M D, Ye P, Firouzi K, et al. Radiation force as a physical mechanism for ultrasonic neurostimulation of the ex vivo retina[J]. The Journal of Neuroscience, 2019, 39(32): 6251-6264.
[111] Cheng Z Y, Wang C M, Wei B W, et al. High resolution ultrasonic neural modulation observed via in vivo two-photon calcium imaging[J]. Brain Stimulation, 2022, 15(1): 190-196.
[112] Lee W, Kim H C, Jung Y, et al. Transcranial focused ultrasound stimulation of human primary visual cortex[J]. Scientific Reports, 2016, 6: 34026.
[113] Yu K, Liu C, Niu X D, et al. Transcranial focused ultrasound neuromodulation of voluntary movement-related cortical activity in humans[J]. IEEE Transactions on Bio-Medical Engineering, 2021, 68(6): 1923-1931.
[114] Kim S, Jo Y, Kook G, et al. Transcranial focused ultrasound stimulation with high spatial resolution[J]. Brain Stimulation, 2021, 14(2): 290-300.
[115] Omar M, Gateau J, Ntziachristos V. Raster-scan optoacoustic mesoscopy in the 25-125 MHz range[J]. Optics Letters, 2013, 38(14): 2472-2474.
[116] Conjusteau A, Ermilov S A, Su R, et al. Measurement of the spectral directivity of optoacoustic and ultrasonic transducers with a laser ultrasonic source[J]. Review of Scientific Instruments, 2009, 80(9): 093708.
[117] Ocheltree K B, Frizzel L A. Sound field calculation for rectangular sources[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 1989, 36(2): 242-248.
[118] Yamashita K, Katata H, Okuyama M, et al. Arrayed ultrasonic microsensors with high directivity for in-air use using PZT thin film on silicon diaphragms[J]. Sensors and Actuators A: Physical, 2002, 97/98: 302-307.
[119] Colchester R J, Little C, Dwyer G, et al. All-optical rotational ultrasound imaging[J]. Scientific Reports, 2019, 9: 5576.
[120] KimJ, ChangW Y, LindseyB D, et al. Laser-generated-focused ultrasound transducers for microbubble-mediated, dual-excitation sonothrombolysis[C]∥ 2016 IEEE International Ultrasonics Symposium (IUS), September 18-21, 2016, Tours, France. New York: IEEE Press, 2016.
[121] Treeby B E, Cox B T. K-Wave: MATLAB toolbox for the simulation and reconstruction of photoacoustic wave fields[J]. Journal of Biomedical Optics, 2010, 15(2): 021314.
[122] Treeby B E, Jaros J, Rendell A P, et al. Modeling nonlinear ultrasound propagation in heterogeneous media with power law absorption using a k-space pseudospectral method[J]. The Journal of the Acoustical Society of America, 2012, 131(6): 4324-4336.
[123] Treeby B E, Cox B T. Modeling power law absorption and dispersion for acoustic propagation using the fractional Laplacian[J]. The Journal of the Acoustical Society of America, 2010, 127(5): 2741-2748.
[124] Lan L, Xia Y, Li R, et al. A fiber optoacoustic guide with augmented reality for precision breast-conserving surgery[J]. Light: Science & Applications, 2018, 7: 2.
[125] Mehić E, Xu J M, Caler C J, et al. Increased anatomical specificity of neuromodulation via modulated focused ultrasound[J]. PLoS One, 2014, 9(2): e86939.
[126] Choi T, Bae S, Suh M, et al. A soft housing needle ultrasonic transducer for focal stimulation to small animal brain[J]. Annals of Biomedical Engineering, 2020, 48(4): 1157-1168.
[127] Arcot Desai S, Gutekunst C A, Potter S M, et al. Deep brain stimulation macroelectrodes compared to multiple microelectrodes in rat hippocampus[J]. Frontiers in Neuroengineering, 2014, 7: 16.
[128] Rosin B, Slovik M, Mitelman R, et al. Closed-loop deep brain stimulation is superior in ameliorating Parkinsonism[J]. Neuron, 2011, 72(2): 370-384.
[129] Krishna V, Sammartino F, Rezai A. A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology: advances in diagnosis and treatment[J]. JAMA Neurology, 2018, 75(2): 246-254.
[130] Roth G A, Mensah G A, Johnson C O, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study[J]. Journal of the American College of Cardiology, 2020, 76(25): 2982-3021.
[131] Virmani R, Burke A P, Farb A, et al. Pathology of the vulnerable plaque[J]. Journal of the American College of Cardiology, 2006, 47(8): C13-C18.
[132] Bode M F, Jaffer F A. IVUS and OCT: current state-of-the-art in intravascular coronary imaging[J]. Current Cardiovascular Imaging Reports, 2019, 12(7): 29.
[133] Ono M, Kawashima H, Hara H, et al. Advances in IVUS/OCT and future clinical perspective of novel hybrid catheter system in coronary imaging[J]. Frontiers in Cardiovascular Medicine, 2020, 7: 119.
[134] Ng A, Swanevelder J. Resolution in ultrasound imaging[J]. Continuing Education in Anaesthesia Critical Care & Pain, 2011, 11(5): 186-192.
[135] Foster F S, Pavlin C J, Harasiewicz K A, et al. Advances in ultrasound biomicroscopy[J]. Ultrasound in Medicine & Biology, 2000, 26(1): 1-27.
[136] Peng C, Wu H Y, Kim S, et al. Recent advances in transducers for intravascular ultrasound (IVUS) imaging[J]. Sensors, 2021, 21(10): 3540.
[137] Mintz G S, Nissen S E, Anderson W D, et al. American college of cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American college of cardiology task force on clinical expert consensus documents developed in collaboration with the European society of cardiology endorsed by the society of cardiac angiography and interventions[J]. European Journal of Echocardiography, 2001, 2(4): 299-313.
[138] Nguyen P, Seto A. Contemporary practices using intravascular imaging guidance with IVUS or OCT to optimize percutaneous coronary intervention[J]. Expert Review of Cardiovascular Therapy, 2020, 18(2): 103-115.
[139] BeyerR T. Nonlinear acoustics: acoustical society of America[M]. New York: American Institute of Physics, 1997.
[140] HamiltonM F, BlackstockD T. Nonlinear acoustics[M]. San Diego: Academic Press, 1998.
[141] Coussios C C, Roy R A. Applications of acoustics and cavitation to noninvasive therapy and drug delivery[J]. Annual Review of Fluid Mechanics, 2008, 40: 395-420.
[142] Herbert E, Balibar S, Caupin F. Cavitation pressure in water[J]. Physical Review E, 2006, 74(4): 041603.
[143] Filonenko E A, Khokhlova V A. Effect of acoustic nonlinearity on heating of biological tissue by high-intensity focused ultrasound[J]. Acoustical Physics, 2001, 47(4): 468-475.
[144] Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications[J]. Nature Reviews Drug Discovery, 2005, 4(3): 255-260.
[145] Choi J J, Selert K, Gao Z M, et al. Noninvasive and localized blood-brain barrier disruption using focused ultrasound can be achieved at short pulse lengths and low pulse repetition frequencies[J]. Journal of Cerebral Blood Flow & Metabolism, 2011, 31(2): 725-737.
[146] Krasovitski B, Frenkel V, Shoham S, et al. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(8): 3258-3263.
[147] Tufail Y, Yoshihiro A, Pati S, et al. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound[J]. Nature Protocols, 2011, 6(9): 1453-1470.
[148] Yoo S S, Bystritsky A, Lee J H, et al. Focused ultrasound modulates region-specific brain activity[J]. NeuroImage, 2011, 56(3): 1267-1275.
[149] Goldenstedt C, Birer A, Cathignol D, et al. Blood clot disruption in vitro using shockwaves delivered by an extracorporeal generator after pre-exposure to lytic agent[J]. Ultrasound in Medicine & Biology, 2009, 35(6): 985-990.
[150] Zhou Y F. High intensity focused ultrasound in clinical tumor ablation[J]. World Journal of Clinical Oncology, 2011, 2(1): 8-27.
[151] Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer[J]. The New England Journal of Medicine, 2002, 347(16): 1233-1241.
[152] van Dongen J A, Voogd A C, Fentiman I S, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European organization for research and treatment of cancer 10801 trial[J]. JNCI: Journal of the National Cancer Institute, 2000, 92(14): 1143-1150.
[153] Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer[J]. The New England Journal of Medicine, 2002, 347(16): 1227-1232.
[154] Siegel R L, Miller K D, Jemal A. Cancer statistics, 2015[J]. CA: A Cancer Journal for Clinicians, 2015, 65(1): 5-29.
[155] Atkins J, Al Mushawah F, Appleton C M, et al. Positive margin rates following breast-conserving surgery for stage I-III breast cancer: palpable versus nonpalpable tumors[J]. Journal of Surgical Research, 2012, 177(1): 109-115.
[156] Balch G C, Mithani S K, Simpson J F, et al. Accuracy of intraoperative gross examination of surgical margin status in women undergoing partial mastectomy for breast malignancy[J]. The American Surgeon, 2005, 71(1): 22-28.
[157] Mitragotri S. Current status and future prospects of needle-free liquid jet injectors[J]. Nature Reviews Drug Discovery, 2006, 5(7): 543-548.
[158] Prausnitz M R, Langer R. Transdermal drug delivery[J]. Nature Biotechnology, 2008, 26(11): 1261-1268.
[159] Torrisi F, Hasan T, Wu W P, et al. Inkjet-printed graphene electronics[J]. ACS Nano, 2012, 6(4): 2992-3006.
[160] Perçin G, Lundgren T S, Khuri-Yakub B T. Controlled ink-jet printing and deposition of organic polymers and solid particles[J]. Applied Physics Letters, 1998, 73(16): 2375-2377.
[161] Han T H, Yoh J J. A laser based reusable microjet injector for transdermal drug delivery[J]. Journal of Applied Physics, 2010, 107(10): 103110.
[162] Fletcher D A, Palanker D V. Pulsed liquid microjet for microsurgery[J]. Applied Physics Letters, 2001, 78(13): 1933-1935.
[163] Chen P H, Chen W C, Chang S H. Bubble growth and ink ejection process of a thermal ink jet printhead[J]. International Journal of Mechanical Sciences, 1997, 39(6): 683-695.
[164] Lee T, Baac H W, Ok J G, et al. Nozzle-free liquid microjetting via homogeneous bubble nucleation[J]. Physical Review Applied, 2015, 3(4): 044007.
Article Outline
王磊, 李琳, 赵永文, 马丁昽, 顾瑛, 王璞. 光致超声技术及其应用[J]. 中国激光, 2023, 50(21): 2107105. Lei Wang, Lin Li, Yongwen Zhao, Dinglong Ma, Ying Gu, Pu Wang. Laser-Generated Ultrasound Technology and Its Application[J]. Chinese Journal of Lasers, 2023, 50(21): 2107105.