金属铋纳米颗粒原位修饰碳纳米管促进锂均匀沉积
随着电动汽车和便携式电子产品在全世界快速普及, 开发高能量密度和长循环寿命的二次电池显得尤为重要。然而, 当前锂离子电池(LIBs)的能量密度几乎达到其理论极限[1]。因此, 开发具有更高比容量的新型电极材料迫在眉睫。锂金属负极由于具有高理论比容量(3860 mAh·g-1)和低电化学电位 (-3.04 V (vs SHE))等优势[2], 被誉为锂电池负极材料的“圣杯”, 是下一代锂硫电池[3]和锂空气电池[4]最理想的负极材料。然而, 锂金属负极在反复沉积/剥离的过程中不可避免地会形成锂枝晶和“死锂”[5-6], 这不仅会阻碍锂离子在电极/电解液界面处的快速传输, 导致电池内阻增加、容量快速衰减, 而且锂枝晶还会刺穿隔膜, 造成电池内部短路, 带来安全隐患。此外, 在充放电过程中, 锂金属表面会反复形成不稳定的固体电解质界面(Solid electrolyte interface, SEI)层[7-8], 导致锂离子通量不均匀, 进一步加剧锂枝晶生长, 并且损耗电解液。这些问题最终将导致锂金属电池(LMBs) 的库仑效率较低和循环稳定性较差, 使其实际应用受到严重阻碍。
近年来, 研究者们开发了多种策略来抑制锂枝晶, 以提高锂金属电池的电化学性能。这些策略主要包括: (1)人工修饰SEI膜[9-10]。SEI膜在锂金属电池中起着至关重要的作用, 它可以阻隔负极与电解液的直接接触, 避免发生副反应。因此, 在锂金属负极表面原位修饰杨氏模量高的人工SEI膜作为保护层, 是抑制枝晶生长和缓解锂金属体积膨胀的有效方法。(2)隔膜改性[11]。隔膜是影响电池性能和寿命的关键部件, 研究表明, 在商业隔膜表面修饰功能化材料可使锂离子通量更加均匀, 有效抑制锂枝晶的形成。(3)电解液设计[12]。电解液和锂金属负极发生电化学反应会生成SEI膜, 隔绝活泼的金属锂和电解液, 以免进一步发生副反应。电解液中引入硝酸锂(LiNO3)和氟化碳酸乙烯酯等添加剂, 可以在锂金属负极表面形成富含氮化锂或氟化锂的SEI膜, 起到抑制锂枝晶生长的作用。(4)集流体的功能化。设计表面亲锂性的三维集流体作为储存锂金属的宿主, 可以抑制锂金属负极枝晶生长和体积膨胀。三维集流体较大的比表面积可以有效降低电极的局部电流密度, 调节电场分布, 从而减缓锂枝晶生长[13]。但三维集流体还有很多问题有待解决, 如孔径大小不一、骨架表面电流分布不均等, 这些因素会导致锂不均匀沉积和锂枝晶生长。此外, 三维集流体相比于二维集流体具有更大的密度, 应用于锂金属电池中会严重降低电池整体的能量密度[14]。因此, 修饰密度更小的二维铜箔作为锂金属负极的集流体, 有望获得高能量密度的锂金属 电池。
商业铜箔的“疏锂”性质不利于锂离子的成核和均匀分布, 易形成锂枝晶, 从而阻碍其在锂金属电池的直接应用[15-16]。因此, 在铜箔表面修饰一层“亲锂”性材料可以使其具有亲锂特性, 降低锂的成核过电势, 实现锂离子通量的均匀分布[17-18]。但修饰过程目前已有的报道大多需要使用复杂的工艺和昂贵的设备, 如化学气相沉积[19]或磁控溅射设备[20]等。因此, 迫切需要开发一种简易的策略在铜箔表面构建亲锂层来促进锂的均匀沉积, 提高锂金属负极的电化学性能。
鉴于此, 本研究发展了一种简单温和的策略, 在碳纳米管表面修饰铋纳米颗粒, 并将所得材料涂覆在商业铜箔表面, 用作锂金属负极的集流体来抑制锂枝晶生长。金属铋和金属锂可以形成合金, 从而降低锂的成核过电势, 促进锂均匀沉积。同时, 相互交织的碳纳米管可为金属锂的沉积提供空间, 使得锂沉积更加均匀致密。最后, 本课题组通过组装锂铜电池和对称电池, 研究了金属锂在不同集流体上沉积/剥离的可逆性和稳定性; 并将预沉积金属锂的复合负极应用于全电池, 考察了改性铜集流体实际应用的可行性。
1 实验方法
1.1 材料合成
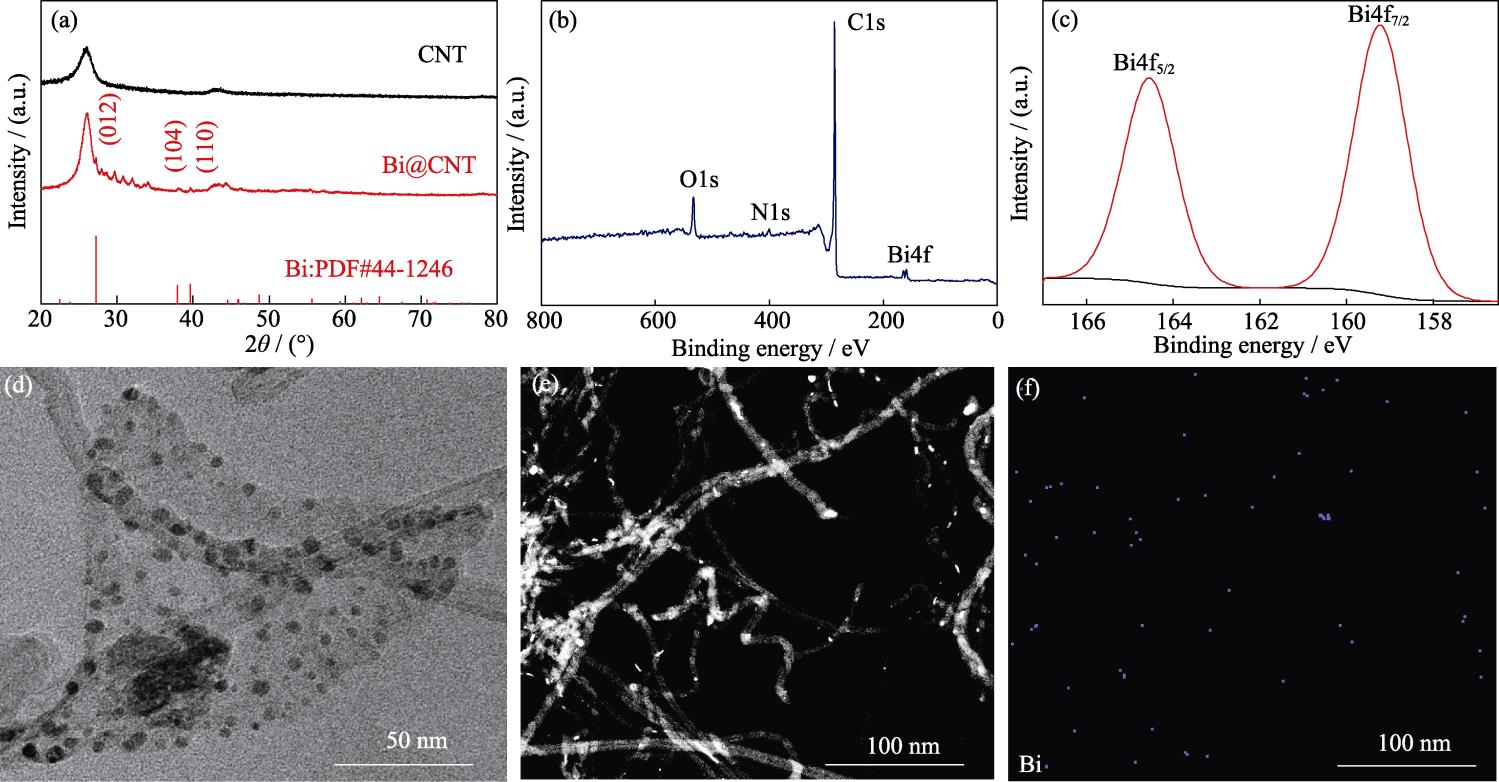
铋纳米颗粒原位修饰碳纳米管(Bi@CNT)的制备流程如
1.2 结构和形貌表征
采用扫描电子显微镜(Scanning electron microscope, SEM, JEOL 4800, 日本)和配备有能量色散X射线(Energy-dispersive X-ray spectroscope, EDS)光谱仪的透射电子显微镜(Transmission electron microscope, TEM, JEOL JEM 2100F, 日本)观察样品的微观形貌和元素分布。采用X射线衍射仪(X-ray diffraction, XRD Bruker D8, 德国, 石墨单色CuKα辐射, λ= 0.15405 nm) 测试样品的晶体结构。采用X射线光电子能谱仪(X-ray photoelectron spectroscope, XPS, Thermo ESCALAB 250, 美国, 单色AlKα)测试样品的元素组成和价态, 以C1s的结合能284.8 eV进行校准。
1.3 电化学测试
在充满氩气的手套箱(H2O和O2含量均低于10-7)中组装CR2032型纽扣电池, 电解液用量为50 μL。采用电池测试系统(LAND CT2001A, 中国)测试所有电池的电化学性能。采用电化学工作站(Chenhua CHI600, 中国)测试电池的电化学阻抗谱(Electrochemical impedance spectroscopy, EIS), 频率范围为0.01 Hz~100 kHz。
2 结果与讨论
2.1 结构和形貌表征
2.2 电化学性能测试
为了探究所得材料对锂枝晶生长的抑制作用和锂均匀沉积的促进作用, 将Bi@CNT与PVDF黏结剂混合均匀制备成浆料, 涂覆在Cu集流体上。以Bi@CNT/Cu为工作电极, 锂箔为对电极组装成锂铜电池, 并测试其库仑效率。对照组采用CNT/Cu和Cu作为工作电极。库仑效率(Coulombic efficiency, CE)是评估锂金属负极循环可逆性的重要参数, 定义为锂沉积量与剥离量的比值。当电流密度为 1 mA·cm-2, 面积容量为1 mAh·cm-2时, 以Bi@CNT/Cu为集流体的锂铜电池循环300圈后库仑效率依旧在98%左右, 库仑效率和循环稳定性明显优于以CNT/Cu和Cu为集流体的锂铜电池, 表明Bi@CNT材料可以提升锂金属沉积/剥离的可逆性与稳定性(
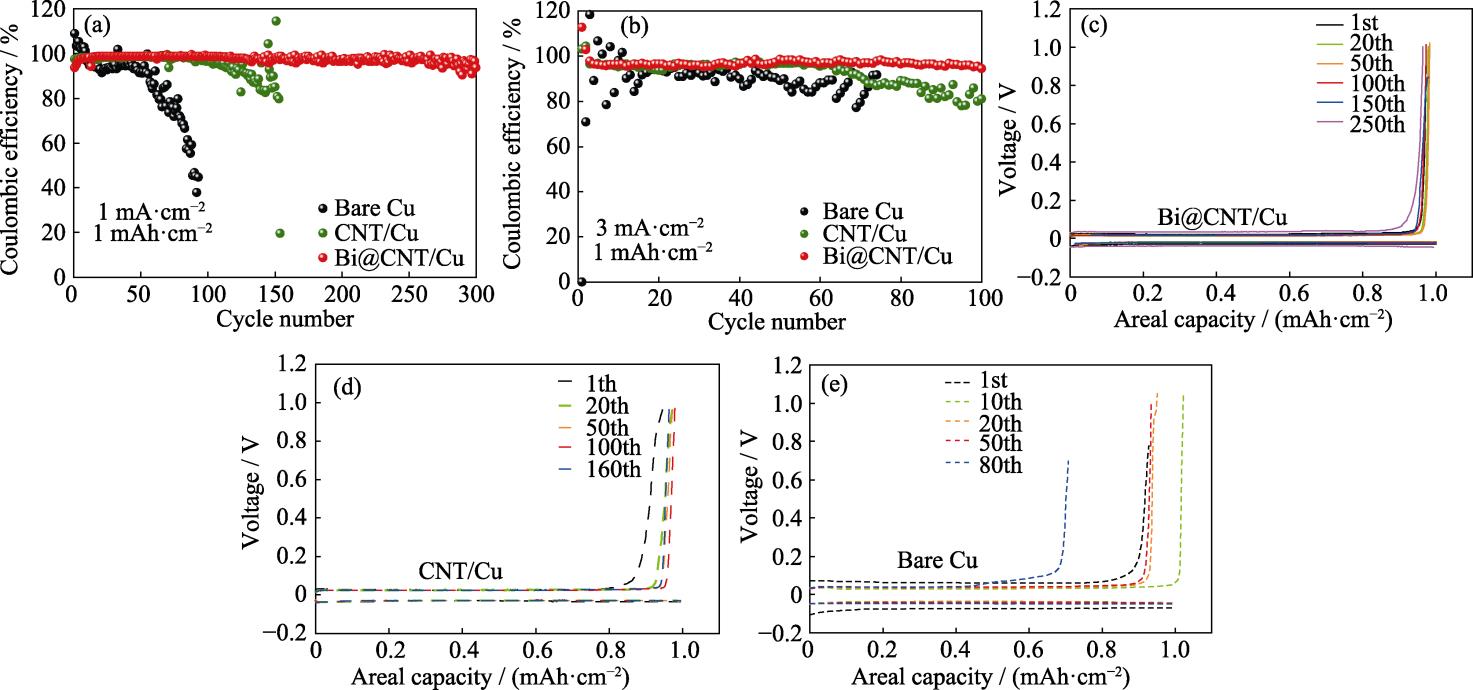
图 3. 基于Bi@CNT/Cu、CNT/Cu和Cu集流体的锂铜电池在(a)1 mA·cm-2, 1 mAh·cm-2和(b)3 mA·cm-2, 1 mAh·cm-2条件下的库仑效率, 基于(c)Bi@CNT/Cu、(d)CNT/Cu和(e)Cu集流体的锂铜电池在1 mA·cm-2, 1 mAh·cm-2条件下的容量-电压曲线
Fig. 3. Coulombic efficiencies of Li|Cu cells based on Bi@CNT/Cu, CNT/Cu and Cu current collectors at (a) 1 mA·cm-2, 1 mAh·cm-2 and (b) 3 mA·cm-2, 1 mAh·cm-2; Capacity-voltage curves of Li|Cu cells based on (c) Bi@CNT/Cu, (d) CNT/Cu, and (e) Cu current collectors at 1 mA·cm-2, 1 mAh·cm-2
为了探究不同集流体的锂沉积情况, 将3种锂铜电池在1 mA·cm-2电流密度和1 mAh·cm-2面积容量下循环50次后, 取出集流体进行表面形貌观察。如
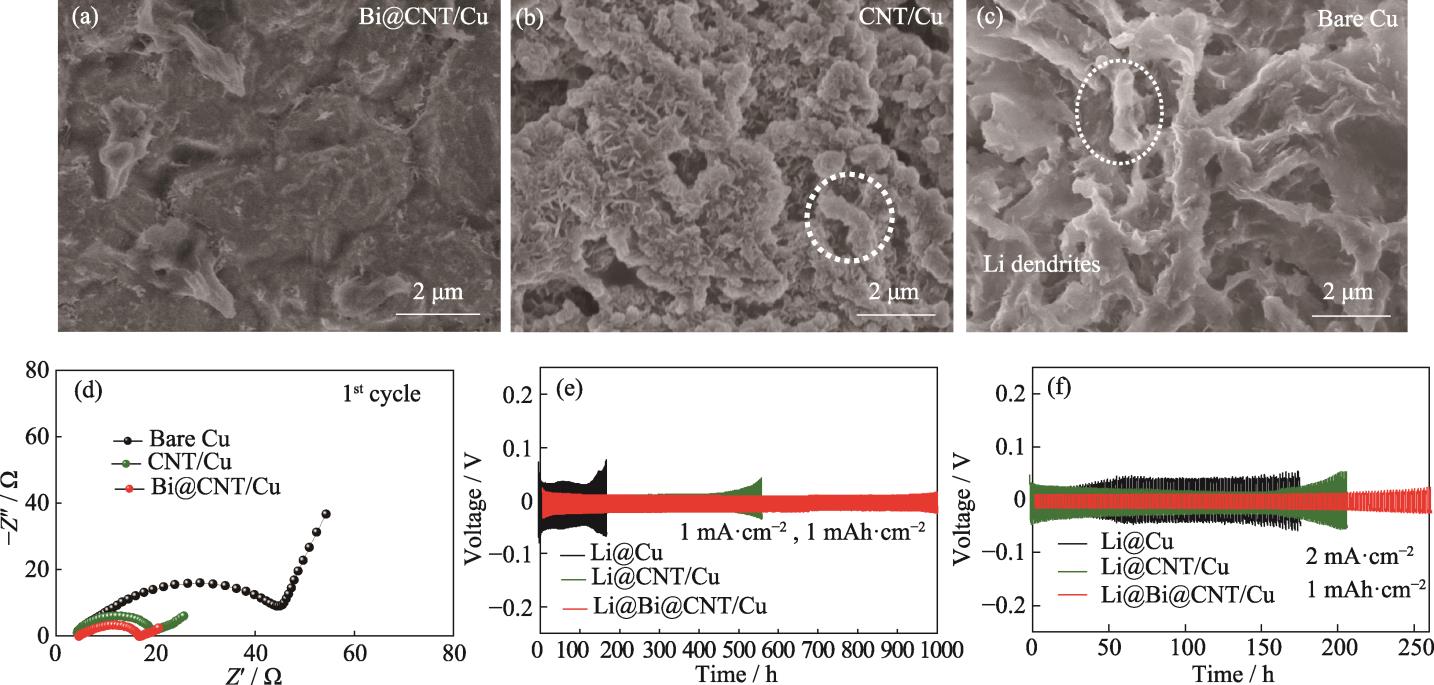
图 4. 锂铜电池循环50次后(a) Bi@CNT/Cu、(b) CNT/Cu和(c) Cu集流体的SEM照片, (d)基于Bi@CNT/Cu、CNT/Cu和Cu集流体的锂铜电池的首圈EIS图谱, 基于Li@Bi@CNT/Cu、Li@CNT/Cu和Li@Cu负极的对称电池在(e)1 mA·cm-2, 1 mAh·cm-2和(f)2 mA·cm-2, 1 mAh·cm-2的电压-时间曲线
Fig. 4. SEM images of (a) Bi@CNT/Cu, (b) CNT/Cu, and (c) Cu current collectors in Li|Cu cells after 50 cycles; (d) First cyclic EIS plots of Li|Cu cells based on Bi@CNT/Cu, CNT/Cu and Cu current collectors, and voltage-time curves of symmetric cells based on Li@Bi@CNT/Cu, Li@CNT/Cu and Li@Cu anodes at (e) 1 mA·cm-2, 1 mAh·cm-2 and (f) 2 mA·cm-2, 1 mAh·cm-2
为了探究不同集流体在长循环过程中对于锂枝晶的抑制作用, 将基于3种集流体的锂铜电池预沉积金属锂形成对称电池后, 进行恒流充放电测试以研究锂的沉积/剥离行为。如
表 1. 使用不同材料修饰铜箔后的电化学性能对比
Table 1. Comparison of electrochemical properties of copper foils modified by different materials
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
为了进一步探究Bi@CNT/Cu集流体实际应用的可能性, 将其组装成全电池进行电化学性能测试。首先将Bi@CNT/Cu集流体预沉积6 mAh·cm-2的金属锂得到复合负极, 再与LFP正极匹配组装成全电池。
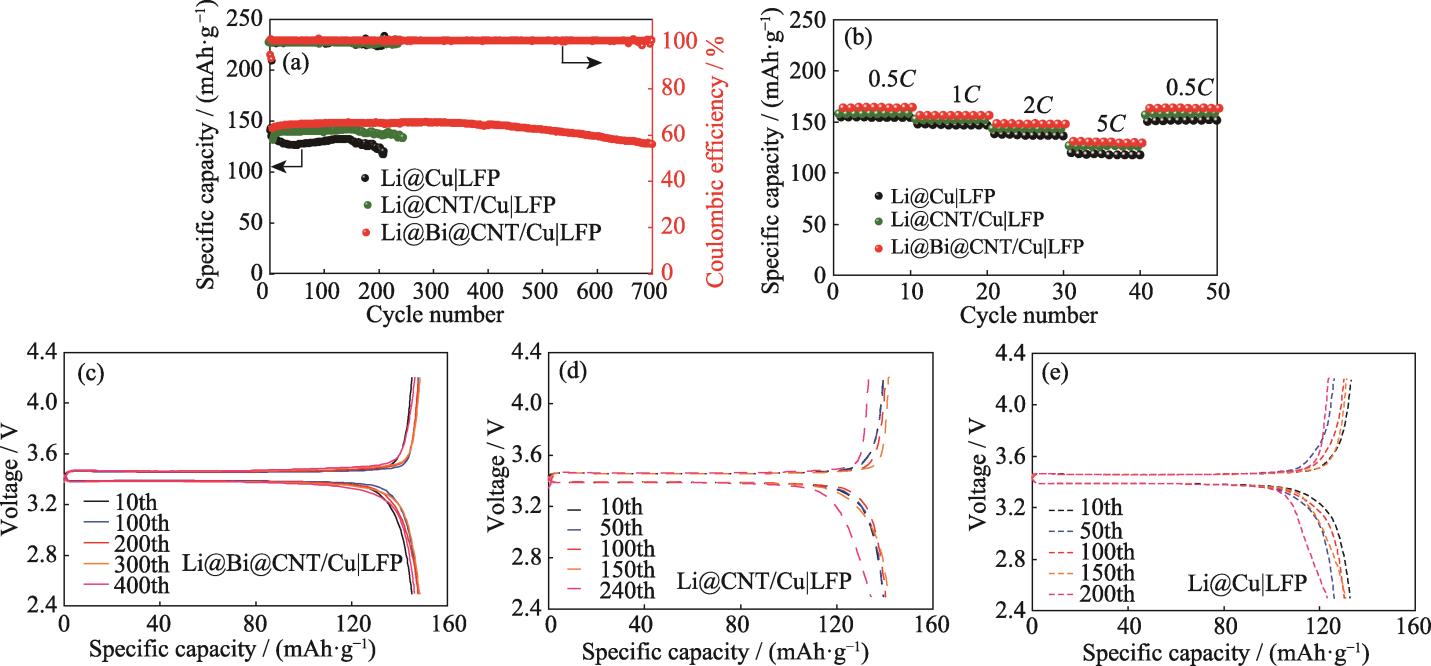
图 5. 基于Li@Bi@CNT/Cu, Li@CNT/Cu和Li@Cu负极的LFP全电池的(a)长循环性能, (b)倍率性能, 基于(c)Li@Bi@CNT/Cu, (d)Li@CNT/Cu, (e)Li@Cu负极的LFP全电池在1C下的容量-电压曲线
Fig. 5. (a) Cycling performances and (b) rate performances of LFP full cells based on Li@Bi@CNT/Cu, Li@CNT/Cu, and Li@Cu anodes, and (c-e) capacity-voltage profiles of LFP full cells based on (c) Li@Bi@CNT/Cu, (d) Li@CNT/Cu, and (e) Li@Cu anodes at 1C
3 结论
本研究通过一种简单温和的方法制备了金属铋纳米颗粒原位修饰的碳纳米管, 并将其涂覆在商业铜箔表面用作锂金属负极的集流体。研究发现, 修饰在碳纳米管上的金属铋可以诱导锂均匀成核, 实现锂均匀沉积, 抑制锂枝晶生长。以Bi@CNT/Cu为集流体的锂铜电池在电流密度为1 mA·cm-2, 容量为 1 mAh·cm-2条件下, 循环300圈后库仑效率仍然在98%, 基于Li@Bi@CNT/Cu负极的对称电池可以稳定循环1000 h。在以磷酸铁锂作为正极材料的全电池中, 预沉积锂的Li@Bi@CNT/Cu负极也表现出优异的循环性能, 在1C条件下可以稳定循环700圈。本研究证明金属铋纳米颗粒原位修饰的碳纳米管可有效促进锂均匀沉积, 为二维铜集流体在锂金属负极中的应用提供了参考。
[5] WOODK N, KAZYAKE, CHADWICKA F, et al.Dendrites and pits: untangling the complex behavior of lithium metal anodes through operando video microscopy. ACS Central Science, 2016, 2(11):790-801. 27924307Enabling ultra-high energy density rechargeable Li batteries would have widespread impact on society. However the critical challenges of Li metal anodes (most notably cycle life and safety) remain unsolved. This is attributed to the evolution of Li metal morphology during cycling, which leads to dendrite growth and surface pitting. Herein, we present a comprehensive understanding of the voltage variations observed during Li metal cycling, which is directly correlated to morphology evolution through the use of operando video microscopy. A custom-designed visualization cell was developed to enable operando synchronized observation of Li metal electrode morphology and electrochemical behavior during cycling. A mechanistic understanding of the complex behavior of these electrodes is gained through correlation with continuum-scale modeling, which provides insight into the dominant surface kinetics. This work provides a detailed explanation of (1) when dendrite nucleation occurs, (2) how those dendrites evolve as a function of time, (3) when surface pitting occurs during Li electrodissolution, (4) kinetic parameters that dictate overpotential as the electrode morphology evolves, and (5) how this understanding can be applied to evaluate electrode performance in a variety of electrolytes. The results provide detailed insight into the interplay between morphology and the dominant electrochemical processes occurring on the Li electrode surface through an improved understanding of changes in cell voltage, which represents a powerful new platform for analysis.
[25] LIUY, WUX, NIUC, et al.Systematic evaluation of carbon hosts for high-energy rechargeable lithium-metal batteries. ACS Energy Letters, 2021, 6(4):1550-1559.
蔡佳, 黄高旭, 金晓盼, 魏驰, 毛嘉毅, 李永生. 金属铋纳米颗粒原位修饰碳纳米管促进锂均匀沉积[J]. 无机材料学报, 2022, 37(12): 1337. Jia CAI, Gaoxu HUANG, Xiaopan JIN, Chi WEI, Jiayi MAO, Yongsheng LI. In-situ Modification of Carbon Nanotubes with Metallic Bismuth Nanoparticles for Uniform Lithium Deposition[J]. Journal of Inorganic Materials, 2022, 37(12): 1337.