基于谱域偏振敏感光学相干层析的局域偏振属性提取及生物组织烧伤深度的定量测量  下载: 625次
下载: 625次
Quantitative measurement of burn depth is of great significance for the clinical assessment of burn degree and treatment plan. Currently, the most widely used assessment method is visual inspection, which places high demands on doctors’ experience and is easily influenced by subjective judgment. Other detection techniques, such as laser Doppler imaging, ultrasound imaging, and fluorescence imaging, have also been used to assess the extent of burns; however, these techniques cannot non-invasively and accurately measure burn depth. Polarization-sensitive optical coherence tomography (PSOCT) has the advantages of non-invasiveness, fast imaging speed and high resolution and can quantitatively measure the burn depth based on the polarization information of the burned tissue. However, the traditional measurement method is based on the accumulated polarization information from the sample surface to a certain depth inside the sample, which cannot accurately characterize the local polarization information at this depth; hence, the burn depth cannot be accurately measured. Therefore, this study proposes a local polarization information extraction algorithm based on spectral domain polarization-sensitive optical coherence tomography (SD-PSOCT) to obtain polarization information at each depth inside the burned biological tissue to quantitatively measure the burn depth of the biological tissue.
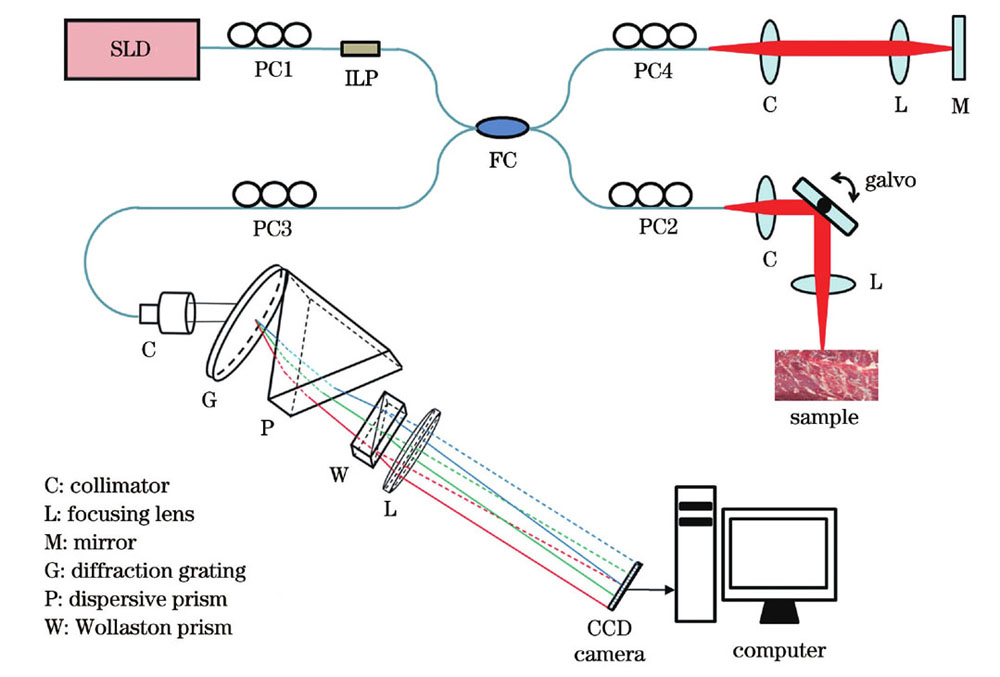
A local polarization property extraction algorithm based on the SD-PSOCT system was proposed and used to quantitatively measure the burn depth of biological tissue. All single-mode-fiber-based systems adopt fiber-based polarization controllers to illuminate a sample with a single-input polarization state. A custom-built linear-in-wavenumber spectrometer consisting of a diffraction grating, dispersive prism, Wollaston prism, and a focusing lens was used to realize polarization-sensitive detection (Fig. 2). Then, the local phase retardation and axis orientation of each layer of the sample were calculated by eigenvalue decomposition based on the Jones matrix and layer-by-layer iterative algorithm. To evaluate the measurement accuracy and stability of the system, we used a quarter-wave plate (QWP) as the sample and measured the phase retardation and axis orientation of the QWP under different axis orientations each day for 14 days. To measure the burn depth of the biological tissue, we selected a piece of bovine tendon tissue as experimental sample, burned the same position of the bovine tendon five times for 10 s each, and then reconstructed the local phase retardation images of the bovine tendon unburned and burned for 10 s, 20 s, 30 s, 40 s, and 50 s, respectively. We then considered the full width at half maximum of the local phase retardation versus the imaging depth curve as the burn depth.
From the sensitivity roll-off curves we can see that the sensitivity at the detection depths of 0.2 mm and 1.2 mm are approximately 105 dB and 98 dB, respectively (Fig. 3). The measured average value of the phase retardation of the QWP is 82.9° and the measurement error is 1.9° (Fig. 4). The 14-day measurement results show that the phase retardation varies within a range of -0.42° to + 0.42° and the axis orientation varies within a range of -0.66° to + 0.66°. By comparing the local phase retardation images of the bovine tendon subjected to different burn times (Fig. 6), it is found that the local phase retardation inside the burned bovine tendon increases, and as the burn time increases, the region with a higher local phase retardation extendes to a deeper position. From the depth-resolved local phase retardation, it can be seen that the region with higher phase retardation gradually widens with increasing burn time (Fig. 6). Thus, the measured burn depth of bovine tendon tissue burned for 50 s is 390 μm.
We deduce the local polarization property extraction algorithm based on the Jones matrix in detail and provide the calculation formulas of local phase retardation and axis orientation. The sensitivity roll-off curves of the two orthogonal polarization channels in the linear wavenumber spectrometer are experimentally measured, and the measured sensitivity of the system is 105 dB. The actual phase retardation and axis orientation of the QWP at different axis orientations are measured and it is verified that the system can measure the polarization properties of birefringent samples with high accuracy and maintain good measurement stability. The imaging results of bovine tendon tissue subjected to different burn times show that the SD-PSOCT system can obtain polarization images with higher contrast than traditional OCT images. Additionally, compared with the cumulative phase retardation image, the local phase retardation image obtained by the algorithm can highlight the difference in the bovine tendon after being burned for different times and quantitatively measure the burn depth according to the local phase retardation images. This study provides a new method for quantitatively measuring tissue burn depth, which can be applied to clinical diagnosis and burn treatment in the future.
1 引言
组织热损伤的测定对于烧伤程度评估及烧伤治疗方案的制定具有重要意义[1]。目前,临床上应用最广泛的烧伤程度评估方法是目视检测[2],即根据烧伤的纹理和颜色以及受烧伤影响的区域大小来确定烧伤程度。该方法对临床医生的经验要求较高,而且评估结果易受主观因素影响[3]。国内外研究小组已经开发出了很多用于客观评估烧伤程度的生物医学成像和检测技术,其中:作为评估烧伤程度标准的组织活检术[4-5]需要从患者体内取出病变组织制作切片,无法实现在体活检;激光多普勒成像[6-7]可以检测血流速度,但无法获取三维信息且容易受到组织厚度和水肿等的影响;超声成像[8-9]与磁共振成像[10]虽然具有厘米级的探测范围,但分辨率较低,而且准确性易受操作者的影响;荧光成像[11]受荧光蛋白种类的限制,较难在体内精确测量烧伤深度。偏振敏感光学相干层析成像(PSOCT)作为OCT的一种功能扩展,除了具有非侵入、无辐射、无损伤、高分辨率等优势[12-13],还可以实现对生物组织内部双折射属性的深度分辨成像,目前已被应用于眼科[14]、口腔科[15-17]等的临床诊断上。事实上,生物组织被烧伤后双折射属性会发生改变,因此,PSOCT也可以用于生物组织热损伤的测定[18-20]。
早期,研究人员[21-24]采用时域空间型PSOCT系统对生物组织的热损伤进行检测,证明了双折射是一种比背向散射强度更灵敏的热损伤指标,但时域OCT成像速度慢,而且其空间型结构不利于紧凑化。之后,研究人员提出了光纤型傅里叶域PSOCT系统,该系统具有成像速度快、结构紧凑、可与内窥探头兼容的优点,更适合于实际临床应用[25]。基于保偏光纤的PSOCT系统能减小光纤扰动对偏振态的影响,但保偏光纤存在偏振模式色散,并且在光纤耦合器处正交偏振光之间会发生交叉耦合,导致图像产生伪影[26-27]。Baumann等[28]和Wang等[29]提出了基于深度复用的扫频PSOCT(SS-PSOCT)系统,该系统使用两束正交偏振光照射样品,实现了单模光纤的偏振敏感成像。Jaspers等[30]采用PSOCT系统在临床环境中评估了人体烧伤瘢痕。基于深度复用的SS-PSOCT方法会牺牲一半的成像深度,且增加了系统的复杂性。Trasischker等[31]提出了基于全单模光纤的SS-PSOCT系统,该系统通过校准偏振控制器得到圆偏振态来照射样品。这些基于扫频光源的系统提高了系统的成本。Kim等[20]和Park等[32]提出了基于单模光纤的谱域PSOCT(SD-PSOCT)系统,该系统采用双光谱仪分别探测两个正交偏振通道的干涉光谱信号,对人体烧伤皮肤的深层和浅表层分别进行定性和定量评估,但是该系统需要额外的触发信号来确保两个光谱仪的光谱响应和时间响应同步。基于单光谱仪的光纤型SD-PSOCT系统[33-36]结构简单紧凑,能实现无伪影的偏振敏感成像,但目前基于单光谱仪的SD-PSOCT系统使用的都是非线性波数光谱仪,相机像素集成的光谱波段在波数域不等间隔;而且为了进行傅里叶变换从而重建图像,干涉光谱信号需要插值重采样至线性波数域,导致计算量增加,并引入了插值误差,降低了对生物组织成像的灵敏度。基于衍射光栅和色散棱镜的线性波数分光能力和Wollaston棱镜的偏振分光特性,研究人员提出了偏振敏感探测型线性波数光谱仪[37],并采用该光谱仪实现了对生物组织的高灵敏度偏振敏感OCT成像。另外,生物烧伤组织的偏振敏感图像大部分是基于双程累积双折射信息获得的,无法真实地表征生物组织某一深度处的局域偏振属性,很可能会影响对烧伤程度的判断。所查资料显示,目前还没有用局域偏振属性检测烧伤深度的相关报道。
本文基于全单模光纤、单输入偏振态和单线性波数光谱仪的SD-PSOCT系统,发展了局域偏振属性提取算法,用于测量生物组织的烧伤深度;测量烧伤生物组织的局域双折射属性,并将其与正常生物组织的局域双折射属性进行对比,实现了烧伤深度的测量。测量系统两个正交偏振通道的灵敏度都达到了83 dB以上。采用该系统对已知偏振特性的偏振器件进行成像,定量评估了该系统的偏振探测性能和稳定性。对经过不同灼烧时间的牛腱组织进行偏振敏感成像实验,分别获得了烧伤牛腱组织的累积和局域偏振敏感图像,研究了本系统对不同烧伤程度生物组织双折射变化的探测能力;同时根据得到的局域相位延迟图像定量地确定了烧伤深度,验证了本系统在评估烧伤深度上的临床应用潜力。
2 方法
基于全单模光纤、单输入偏振态和单线性波数光谱仪的SD-PSOCT系统的原理如
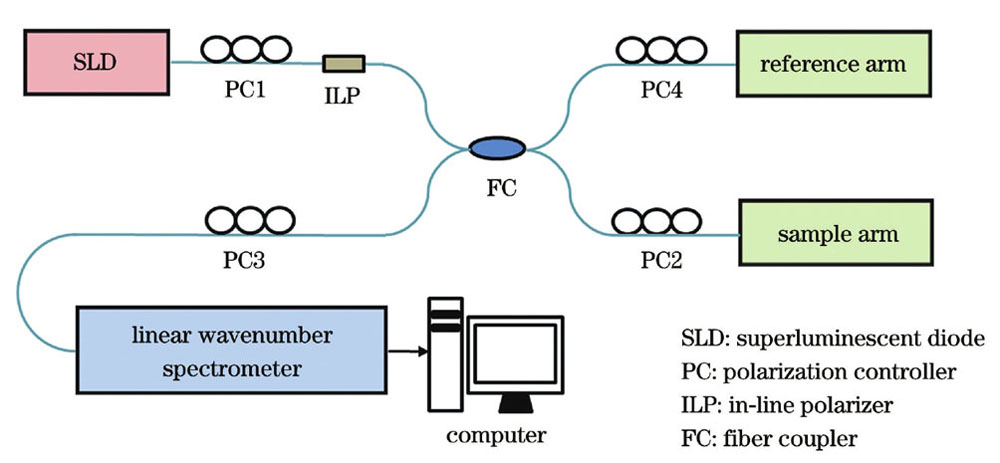
图 1. 基于全单模光纤、单输入偏振态和单线性波数光谱仪的SD-PSOCT的原理图
Fig. 1. Principle of proposed SD-PSOCT based on all single-mode fiber, single input polarization state and single linear wavenumber spectrometer
调节PC2,使照射样品的入射光为右旋圆偏振态,其琼斯矢量可表示为
正交偏振通道(水平H通道和垂直V通道)中的干涉光谱信号的干涉项可表示为
通过对两个正交偏振通道中的干涉光谱信号
通过式(8)和式(9)计算的累积相位延迟和累积光轴方位角无法反映样品某深度z处的局域偏振属性。在系统轴向分辨单元内的样品薄层的光轴方位角可以看作是均匀的,所以每一个薄层可以等效为形如式(1)的延迟器。为了提取样品某深度z处的局域偏振属性,设
3 实验
所提SD-PSOCT系统示意图如
4 实验结果
4.1 系统性能测量结果
为了测试系统的灵敏度,以平面镜为样品,改变参考臂与样品臂之间的光程差,采集对应不同探测深度的两正交偏振通道的干涉光谱信号,经直流项去除和傅里叶逆变换后得到对应不同探测深度的点扩散函数。
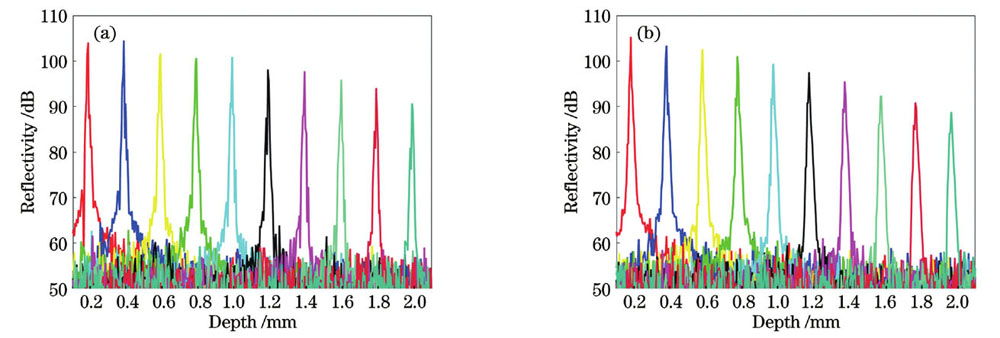
图 3. SD-PSOCT系统的灵敏度测量。(a)水平偏振通道;(b)竖直偏振通道
Fig. 3. Sensitivity measurement of SD-PSOCT system. (a) Horizontal polarization channel; (b) vertical polarization channel
为了测试系统对相位延迟和光轴方位角的测量精度,以四分之一波片(GCL-060802)为样品,测量其在不同光轴方位角下的相位延迟和光轴方位角。测量结果如
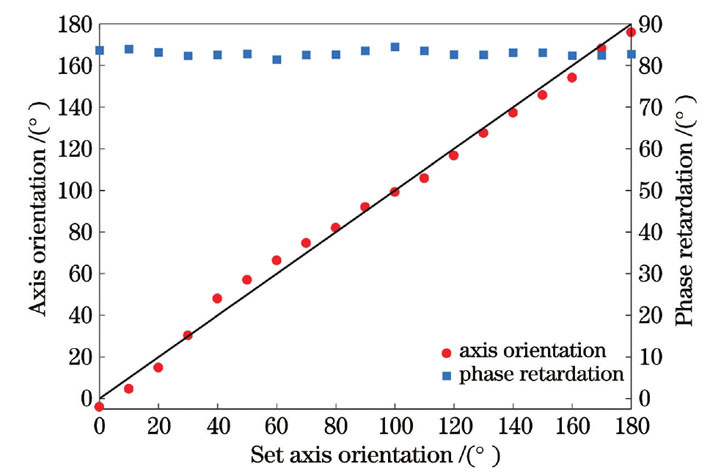
图 4. 在不同光轴方位角情况下测得的四分之一波片的相位延迟和光轴方位角
Fig. 4. Measured phase retardation and axis orientation values of quarter-wave plate under different axis orientations
为进一步定量评估系统的性能稳定性,在14 d内每天测量未受环境影响的同一块四分之一波片的相位延迟和光轴方位角。实验结果显示,系统测量的14次相位延迟变化范围为-0.42°~+0.42°,光轴方位角的变化范围为-0.66°~+0.66°。这说明该系统具有较好的稳定性,能够保持长时间免调试地高精度测量双折射样品的偏振属性。
4.2 烧伤组织成像结果
为了验证本系统在定量测量生物组织烧伤深度上的实际应用效果,选用一块新鲜的离体牛腱组织作为样品,使用超连续谱光源(SC-PRO)作为灼烧激光多次照射牛腱表面同一位置,通过累积灼烧时间改变烧伤程度,对不同烧伤程度的牛腱组织进行PSOCT成像。每次照射功率为4 W,照射时间为10 s,照射在牛腱表面上的灼烧激光光斑直径约为2 mm。重建的PSOCT图像如
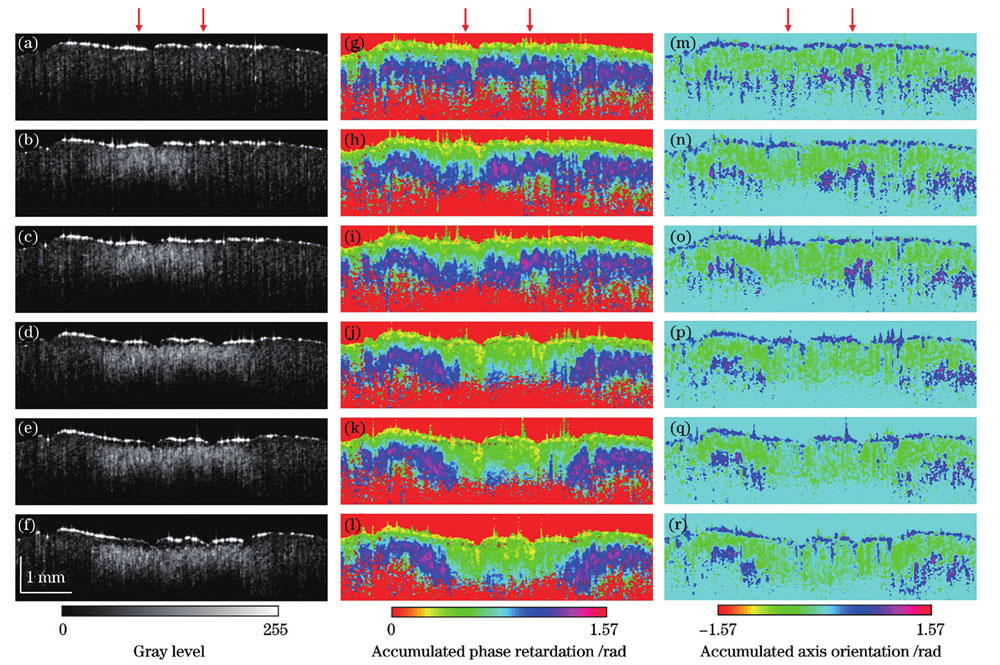
图 5. 未灼烧以及累积灼烧10、20、30、40、50 s牛腱组织的PSOCT图像重建结果。(a)~(f)OCT强度图像;(g)~(l)累积相位延迟层析图像;(m)~(r)累积光轴方位角层析图像
Fig. 5. Reconstructed PSOCT images of unburned bovine tendon tissue and burned bovine tendon tissue with burning time of 10, 20, 30, 40, and 50 s, respectively. (a)-(f) OCT intensity images; (g)-(l) cumulative phase retardation tomography images; (m)-(r) cumulative axis orientation tomography images
基于上文阐述的局域偏振属性提取算法,重建得到了局域偏振属性图像,如

图 6. 未灼烧以及累积灼烧10、20、30、40、50 s牛腱组织的重建局域偏振属性图像。(a)~(f)局域相位延迟层析图像;(g)~(l)局域光轴方位角层析图像;(m)局域相位延迟平均值随成像深度的变化;(n)烧伤深度随灼烧时间的变化
Fig. 6. Reconstructed images of local polarization properties of unburned bovine tendon tissue and burned bovine tendon tissue with burning time of 10, 20, 30, 40, and 50 s, respectively. (a)-(f) Local phase retardation tomography images; (g)-(l) local axis orientation tomography images; (m) average local phase retardation varied with imaging depth; (n) burn depth varied with burning time
5 结论
本团队采用基于SD-PSOCT系统的局域偏振属性提取算法定量测量了生物组织的烧伤深度。该系统是紧凑的全单模光纤型结构,使用单输入偏振态照射样品,使用单线性波数光谱仪对样品进行偏振敏感探测。本文详细推导并介绍了基于琼斯矩阵的局域偏振属性提取算法,给出了局域相位延迟和光轴方位角的计算公式。实验测得了线性波数光谱仪中两个正交偏振通道的灵敏度下降曲线,其在零光程附近的灵敏度达到了105 dB。测量了不同光轴方位角下四分之一波片的实际相位延迟和光轴方位角,验证了该系统能高精度地测定双折射样品的偏振属性,同时整个系统能保持良好的稳定性。不同灼烧时间的牛腱组织的成像结果显示了该SD-PSOCT系统可以获取比传统OCT强度图像更具对比度的偏振图像,而采用局域偏振属性提取算法获取的局域相位延迟层析图像比累积相位延迟层析图像更能凸显牛腱组织在经历不同时间灼烧后的区别。根据局域相位延迟层析图像定量测量了不同灼烧时间对应的烧伤深度。本研究为组织烧伤深度测量提供了新方法和定量测量依据,今后有望应用于烧伤的临床诊断与治疗。
[1] Monstrey S, Hoeksema H, Verbelen J, et al. Assessment of burn depth and burn wound healing potential[J]. Burns, 2008, 34(6): 761-769.
[2] Sheridan R L. Comprehensive treatment of burns[J]. Current Problems in Surgery, 2001, 38(9): 657-756.
[3] Pierce M C, Sheridan R L, Park B H, et al. Collagen denaturation can be quantified in burned human skin using polarization-sensitive optical coherence tomography[J]. Burns, 2004, 30(6): 511-517.
[4] Ho-Asjoe M, Chronnell C M, Frame J D, et al. Immunohistochemical analysis of burn depth[J]. The Journal of Burn Care & Rehabilitation, 1999, 20(3): 207-211.
[5] Watts A M I, Tyler M P H, Perry M E, et al. Burn depth and its histological measurement[J]. Burns, 2001, 27(2): 154-160.
[6] Holland A J A, Martin H C O, Cass D T. Laser Doppler imaging prediction of burn wound outcome in children[J]. Burns, 2002, 28(1): 11-17.
[7] Jeng J C, Bridgeman A, Shivnan L, et al. Laser Doppler imaging determines need for excision and grafting in advance of clinical judgment: a prospective blinded trial[J]. Burns, 2003, 29(7): 665-670.
[8] Iraniha S, Cinat M E, VanderKam V M, et al. Determination of burn depth with noncontact ultrasonography[J]. The Journal of Burn Care & Rehabilitation, 2000, 21(4): 333-338.
[9] Bauer J A, Sauer T. Cutaneous 10 MHz ultrasound B scan allows the quantitative assessment of burn depth[J]. Burns, 1989, 15(1): 49-51.
[10] Nettelblad H, Thuomas K Å, Sjöberg F. Magnetic resonance imaging: a new diagnostic aid in the care of high-voltage electrical burns[J]. Burns, 1996, 22(2): 117-119.
[11] Still J M, Law E J, Klavuhn K G, et al. Diagnosis of burn depth using laser-induced indocyanine green fluorescence: a preliminary clinical trial[J]. Burns, 2001, 27(4): 364-371.
[12] 刘颖, 杨亚良, 岳献. 光学相干层析血管造影术及其在眼科学中的应用[J]. 激光与光电子学进展, 2020, 57(18): 180002.
[13] 薛平. 高性能光学相干层析成像的研究[J]. 中国激光, 2021, 48(15): 1517001.
[14] Cense B, Chen T C, Park B H, et al. In vivo depth-resolved birefringence measurements of the human retinal nerve fiber layer by polarization sensitive optical coherence tomography[J]. Optics Letters, 2002, 27(18): 1610-1612.
[15] Wang X J, Milner T E, de Boer J F, et al. Characterization of dentin and enamel by use of optical coherence tomography[J]. Applied Optics, 1999, 38(10): 2092-2096.
[16] Golde J, Tetschke F, Walther J, et al. Detection of carious lesions utilizing depolarization imaging by polarization sensitive optical coherence tomography[J]. Journal of Biomedical Optics, 2018, 23(7): 071203.
[17] 胡慕芸, 杨迪, 杨子晗, 等. 基于偏振敏感光学相干层析术的口腔鳞状细胞癌组织成像[J]. 光学学报, 2022, 42(10): 1017002.
[18] Park B H, Saxer C E, Srinivas S M, et al. In vivo burn depth determination by high-speed fiber-based polarization sensitive optical coherence tomography[J]. Journal of Biomedical Optics, 2001, 6(4): 474-479.
[19] Srinivas S M, de Boer J F, Park B H, et al. Determination of burn depth by polarization-sensitive optical coherence tomography[J]. Journal of Biomedical Optics, 2004, 9(1): 207-212.
[20] Kim K H, Pierce M C, Maguluri G N, et al. In vivo imaging of human burn injuries with polarization-sensitive optical coherence tomography[J]. Journal of Biomedical Optics, 2012, 17(6): 066012.
[21] Schoenenberger K, Colston B W, Maitland D J, et al. Mapping of birefringence and thermal damage in tissue by use of polarization-sensitive optical coherence tomography[J]. Applied Optics, 1998, 37(25): 6026-6036.
[22] de Boer J F, Srinivas S M, Malekafzali A, et al. Imaging thermally damaged tissue by polarization sensitive optical coherence tomography[J]. Optics Express, 1998, 3(6): 212-218.
[23] Jiao S L, Yu W R, Stoica G, et al. Contrast mechanisms in polarization-sensitive Mueller-matrix optical coherence tomography and application in burn imaging[J]. Applied Optics, 2003, 42(25): 5191-5197.
[24] Todorović M, Jiao S L, Ai J, et al. In vivo burn imaging using Mueller optical coherence tomography[J]. Optics Express, 2008, 16(14): 10279-10284.
[25] Ding Z Y, Liang C P, Tang Q G, et al. Quantitative single-mode fiber based PS-OCT with single input polarization state using Mueller matrix[J]. Biomedical Optics Express, 2015, 6(5): 1828-1843.
[26] Davé D P, Akkin T, Milner T E. Polarization-maintaining fiber-based optical low-coherence reflectometer for characterization and ranging of birefringence[J]. Optics Letters, 2003, 28(19): 1775-1777.
[27] Götzinger E, Baumann B, Pircher M, et al. Polarization maintaining fiber based ultra-high resolution spectral domain polarization sensitive optical coherence tomography[J]. Optics Express, 2009, 17(25): 22704-22717.
[28] Baumann B, Choi W, Potsaid B, et al. Swept source/Fourier domain polarization sensitive optical coherence tomography with a passive polarization delay unit[J]. Optics Express, 2012, 20(9): 10229-10241.
[29] Wang Z, Lee H C, Ahsen O O, et al. Depth-encoded all-fiber swept source polarization sensitive OCT[J]. Biomedical Optics Express, 2014, 5(9): 2931-2949.
[30] Jaspers M E H, Feroldi F, Vlig M, et al. In vivo polarization-sensitive optical coherence tomography of human burn scars: birefringence quantification and correspondence with histologically determined collagen density[J]. Journal of Biomedical Optics, 2017, 22(12): 121712.
[31] Trasischker W, Zotter S, Torzicky T, et al. Single input state polarization sensitive swept source optical coherence tomography based on an all single mode fiber interferometer[J]. Biomedical Optics Express, 2014, 5(8): 2798-2809.
[32] Park B H, Pierce M C, Cense B, et al. Real-time fiber-based multi-functional spectral-domain optical coherence tomography at 1.3 μm[J]. Optics Express, 2005, 13(11): 3931-3944.
[33] Baumann B, Götzinger E, Pircher M, et al. Single camera based spectral domain polarization sensitive optical coherence tomography[J]. Optics Express, 2007, 15(3): 1054-1063.
[34] Cense B, Mujat M, Chen T C, et al. Polarization-sensitive spectral-domain optical coherence tomography using a single line scan camera[J]. Optics Express, 2007, 15(5): 2421-2431.
[35] Lee S W, Jeong H W, Kim B M. High-speed spectral domain polarization-sensitive optical coherence tomography using a single camera and an optical switch at 1.3 μm[J]. Journal of Biomedical Optics, 2010, 15(1): 010501.
[36] Fan C M, Wang Y, Wang R K. Spectral domain polarization sensitive optical coherence tomography achieved by single camera detection[J]. Optics Express, 2007, 15(13): 7950-7961.
[37] Wu T, Cao K M, Wang X H, et al. Single input state, single mode fiber based spectral domain polarization sensitive optical coherence tomography using a single linear-in-wavenumber spectral camera[J]. Optics and Lasers in Engineering, 2020, 127: 105948.
[38] Fan C M, Yao G. Mapping local retardance in birefringent samples using polarization sensitive optical coherence tomography[J]. Optics Letters, 2012, 37(9): 1415-1417.
[39] Fan C M, Yao G. Mapping local optical axis in birefringent samples using polarization-sensitive optical coherence tomography[J]. Journal of Biomedical Optics, 2012, 17(11): 110501.
Article Outline
吴彤, 周鑫康, 刘友文, 王吉明, 路元刚, 沈红, 顾晓蓉, 施瑶瑶, 赫崇君. 基于谱域偏振敏感光学相干层析的局域偏振属性提取及生物组织烧伤深度的定量测量[J]. 中国激光, 2022, 49(24): 2407203. Tong Wu, Xinkang Zhou, Youwen Liu, Jiming Wang, Yuangang Lu, Hong Shen, Xiaorong Gu, Yaoyao Shi, Chongjun He. Local Polarization Properties Extraction Based on Spectral Domain Polarization Sensitive Optical Coherence Tomography and Quantitative Burn Depth Measurement of Biological Tissues[J]. Chinese Journal of Lasers, 2022, 49(24): 2407203.