医用生物陶瓷的功能性生物适配机制及应用
Research on “Biocompatibility” (related to biomaterials) can be traced back to the 1960s[1]. Initially, the definition of biocompatibility was established by consensus, which is the ability of biomaterial to produce an appropriate reaction in a particular application or the interaction between the composition of biomaterials and the local and systemic tissues. But that definition was based on bioinert materials[2]. Therefore, it also became the initial evaluation condition for dense bioceramics at that time. In the 1980s, scholars' understanding of biocompatibility changed. Their emphasis was centered on bioactivity and biointegration. Then, in the 1990s the concept of biocompatibility was further supplemented, including the chemical composition released by the degradation of biological materials, the mechanical strength required for tissue repair, and the structural bionics of repaired tissues[1]. Until the early 2000s, scholars shifted their attention away from biomaterials themselves and toward practical application needs[3].
Biomaterials are the product of interdisciplinary research. With the development of various related disciplines (such as immunology, molecular biology and biomechanics), the definition of biocompatibility has become difficult to describe those excellent biomaterials. In addition, as stated by Williams, the definition of biocompatibility has limitations that will not guide researchers in developing biomaterials with excellent biocompatibility[2]. According to this situation, the definition and connotation of “Bioadaptability” have been proposed naturally[4]. It describes the important characteristics of the excellent existing biomaterials and serves as a very demanding benchmark for the selection, design, and evaluation of upcoming biomaterials. In brief, its connotation includes three aspects[4]. Tissue adaptability: the microenvironment generated by the biomaterial can harmonize with the tissue at the implant site. Degradation adaptability: the degradation properties of biomaterials can meet the needs of new tissue formation. Mechanical adaptability: the mechanical properties of biomaterials can meet the needs of tissue defect repair. Based on clinical practical application, “Precise Bioadaptability”, aiming at precision medicine, has been further proposed[5]. Its core is that the ability of biomaterials can create a microenvironment in harmony with a host tissue in situ. So, what requirements of precise bioadaptability should biomaterials possess in the field of bone defect repair and reconstruction?
In terms of microstructure, although the dense structure of medical bioceramics can meet the needs of mechanical support to a certain extent, it is difficult to achieve bone in-growth, resulting in unsatisfying effect of bone repair. If the structure is porous, it can meet the needs of bone in-growth. But its insufficient mechanical properties can greatly and negatively affect the reconstruction and may even totally fail. In addition, if the medical bioceramics degrade too quickly to last long enough for new bone formation, they often collapse, leading to reconstruction failure as well. So, what requirements should medical bioceramics have on structure, degradation, and mechanics?
Regarding those questions, we would like to continue the discussion around the theme “Functional Bioadaptability in medical bioceramics”, this is an experience sharing based on our research findings over the years. The discussion follows the order of structural adaptability, degradation adaptability, mechanical adaptability, and application adaptability of medical bioceramics (mainly calcium phosphate-based ceramics in this perspective). Ultimately, sharing our experience hopes to provide references and proposals for the design, production, and application of upcoming medical bioceramics.
1 Soul: structural adaptability
1.1 Microstructures of bioceramics
The microstructure of medical bioceramics includes grains, macropores, micropores, porosity, internal connections, and their distribution, shape, surface morphology, etc. These parameters can regulate cell adhesion, extension, migration, proliferation, and differentiation. Thus, it affects the process of bone in-growth, which finally determines the outcome of bone defect repair and reconstruction. Consequently, the controllable preparation of bioceramic microstructures is the key to achieving functional bioadaptability in medical bioceramics.
1.2 Research on microstructures of bioceramics
Historically, the academic interest in microstructure stemmed from a biomaterial used in dentistry and plastic surgery (called Cerosium®) in the 1960s[1]. Even if its surface porosity (diameter≤25 μm in cross-section) was proven to be a failure in the subsequent clinical research, it opened the door to the study of pore size (macroporous and microporous) and porous materials[1]. For instance, subsequent research confirmed that the size was supposed to be 75-100 μm for achieving vascularization and bone in-growth stability. But, the so-called porous materials at that time, were not truly porous because of the lack of interconnectivity. Their porous structures are isolated, parallel, and not connected. Inspired by the cancellous bone structure, truly porous materials with microstructural interconnectivity were developed in the 1980s[1]. Gradually, it is realized that interconnectivity is of decisive significance to bone in-growth[1]. However, until the early 21st century, though scholars have recognized the importance of microstructure (such as porosity, pore size, and interconnectivity mentioned above), how these parameters regulate the efficiency of bone repair remains unknown[6].
1.2.1 Research on cell-material-blood supply based on microstructures of bioceramics
Since the beginning of the 21st century, the progress of preparation technology has realized the precise control of these parameters step-by-step. Increasingly scholars have the opportunity to explore the “appropriate” or even “optimal” range of these parameters in the process of bone defect repair[7⇓⇓⇓⇓⇓⇓-14]. At the same time, scholars also realize that bionic anatomy is not the same as bionic repair structure. For instance, imitating the dense structure of cortical bone is not conducive to the repair of cortical bone by bone implants[15]. The author’s team developed a preparation technology of bioceramic with approximately 100% interconnectivity (Chinese Patent CN1268583C and CN101172883B), which could accurately control the macroporous size and interconnection diameter[16].
A series of microstructural parameters were explored around these bioceramics[17]. And the theory of “Cell-Material-Blood supply” interacting with trinity tissue regeneration was further proposed (Fig. 1). To be more specific, cell, material and blood supply are regarded as seeds, soil, and fertilizer, respectively. Soil is the foundation of seed growth, fertilizer is the essential nutrition of seeds, and quality soil plays a key role in helping fertilizer. In other words, angiogenesis is essential for cell and tissue growth. The microstructure of materials as a medium provides favorable three- dimensional space conditions for the growth of blood vessels and the formation of new bone. Only by realizing coordination among the three can the functional bioadaptability be maximized. Moreover, the reason why structural adaptability serves as the soul of functional bioadaptability, is that it determines whether medical bioceramics can achieve in situ adaptability, and also affects the biodegradation process and mechanical support changes in the whole subsequent repair process. The microstructural parameters, such as porosity, pore size, and interconnectivity, are the concrete manifestation of its application.
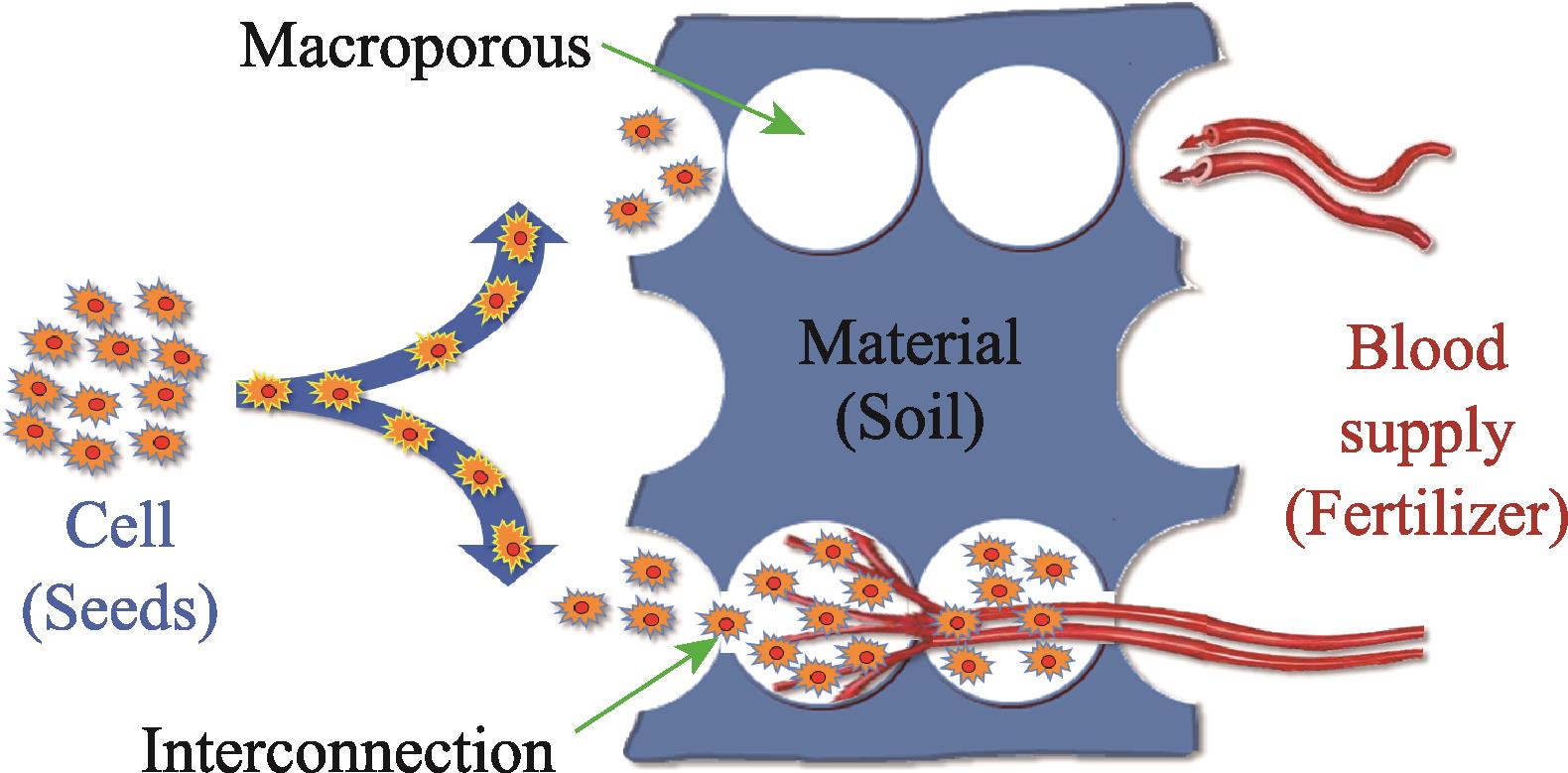
图 1.
Fig. 1. Theory of “Cell-Material-Blood supply” interacting with trinity tissue regeneration[17]
1.2.2 Effect of microstructure on cell recombination and proliferation
The microstructures (porosity, macropore and interconnection) directly affect the transport of oxygen and nutrients in the bioceramics, and thus affect the adhesion, proliferation, and differentiation of cells[18]. But the effect of porosity on cells is different from that of macropore. In vitro, high porosity does not affect cell adherence but can promote cell proliferation. This mainly depends on the increased porosity conducive to the transport of oxygen and nutrients. Low porosity can inhibit cell proliferation, but facilitate cell aggregation and growth to stimulate bone formation. In terms of macroporous and interconnective, the authors also researched the effect of different pore sizes of them respectively on cell recombination and adhesion[17,19]. They found that the initial number of composite cells was consistent with the trend of cell adhesion. Specifically, the amount of cell recombination is inversely proportional to the macroporous size, while the effect of pore size of interconnective is not obvious. Two reasons are mainly considered. Firstly, the porosity of larger pore-size materials is relatively smaller, so the amount of cell suspension entering is smaller. Secondly, under the condition of better interconnective, the liquid siphoning effect of larger pore size is weak, which shortens the residence time after cell adhesion, thus affecting subsequent chain effects such as cell motility and proliferation.
Besides, SEM and histological observations showed that cell growth was observed in all different combinations of the pore size of macroporous and interconnective[17]. With the pore size increasing, the number of cells growing in the interior increased, which was also consistent with the previous research results of the author’s team[19]. Interestingly, the presence of cell layers was observed only in materials with pore sizes of macroporous (500-600 μm and 600-700 μm) and interconnective pores (150 μm)[19]. The histomorphometric results showed that the range of cells that could fully grow and proliferate in the material increased with the increase of pore sizes. There was no difference between cell growth and cell proliferation when the interconnective pore size was 70-120 μm, but the cell growth and proliferation increased significantly when that was more than 150 μm.
1.2.3 Effects of microstructure on cell motility and nutrient supply
Following the above, under static culture conditions, the growth and proliferation of cells in porous material were limited from the first to second rows of pores[20]. To better simulate the changes in cell activity and nutrient supply brought about by fluid flow in the body, the author’s team designed a set of three-dimensional flow dynamic culture system, perfusion bioreactor[21-22]. In this system, nutrients are pumped into liquid storage bottles, and through a system of pipes into the material’s interconnecting macroporous pore structures, allowing cells to adhere and proliferate. In dynamic culture, oxygen exchange and nutrient supply were improved in both the edges and the center of the implant. And metabolic wastes were discharged directly. In these researches, the parameters of the bioceramics were 75% porosity, (530±100) μm macroporous sizes, and (150±50) μm interconnective pore size. Compared with static culture, the glucose consumption and cell proliferation of the cells in dynamic culture were more significant, and the cells could distribute and live in the entire interior of the bioceramics (Fig. 2). Therefore, porous materials with satisfactory interconnectivity are the basis for the wide distribution of seed cells in vivo and the promotion of cell proliferation by the nutrient exchange. Notably, this kind of bioreactor is also one of the ways to build tissue engineered bone. The potential of bone tissue repair can be improved by cell pre-loading and even pre-differentiation of medical bioceramics through that perfusion bioreactor[23⇓⇓-26].

图 2.
Fig. 2. Effects of microstructure on cell recombination and proliferation[21,27](a) Cell motility in bioceramic microstructure with three-dimensional flow dynamic culture system, perfusion bioreactor; (b) SEM images of the cross section of a scaffold seeded with sheep MSCs; (c) Histological section of the cell-TCP composite stained with May-Grünwald Giemsa (A-F)
1.2.4 Effect of microstructure on osteogenesis
The microstructures (macroporous size, interconnectivity, porosity, and shape) are the decisive factors affecting the pattern and amount of bone formations. Porosity is the most basic requirement of bioceramics as medical bioceramics. Previous research has shown that the minimum macroporous size required for cell migration, nutrient input, and metabolite excretion is 100 μm, and the recommended ideal size for osteogenesis is above 300 μm[18]. The research of our team showed that osteoblasts were able to grow and proliferate when the interconnective pore size was less than 20 μm. When pore sizes between 20-50 μm, chondroid and osteoid tissue can be formed, and more than 50 μm can form mineralized bone[19]. We also confirmed that the macroporous size of porous bioceramics directly determines the porosity which is inversely proportional to the macroporous size and directly proportional to the interconnective pore size. The porosity must also meet certain requirements. High porosity can increase cell differentiation, tissue growth, and angiogenesis, but the mechanical properties will also be decreased. When the porosity exceeds 30%, the macropores can reach interconnection, and the new bone tissue can grow from the surface into the interior, forming a network structure. The higher the porosity, the better the new bone growth, and the higher the reconstructed bone strength. To meet the mechanical property requirements of clinical application, the porosity of scaffolds is generally controlled within 40%-60%[17]. In addition, depending on the shape of the bone defect site, the bioceramic should also have certain personalized shape characteristics.
Microstructure also affects the bone formation pattern of bioceramics. Our team considered it may depend on the blood supply. When the macroporous size of porous HA is 90-110 μm, the bone formation pattern is similar to endochondral osteogenesis. When the macroporous size is 350 μm, the bone formation pattern is similar to the intramembrane osteogenesis[28]. By dynamically observing the vascularization of bioceramics with different macroporous sizes, some scholars found that vascularization began within a short time after implantation, and vascularization and osteogenesis were closely related to the macroporous size and interconnective[29]. Vascularization also affects the amount of new bone formation. The blood vessel density with macroporous size >140 μm was significantly higher than that with <140 μm, resulting in significantly higher new bone volume[29]. No cartilage formation was observed during this process. Although there was more blood vessel formation in the material with larger macroporous sizes, the volume of bone formation was less than that in the material with smaller size. This is mainly because the material with small macroporous sizes is more conducive to bone formation and mineral deposition[29-30]. Our team compared porous HA and porous β-TCP with macroporous sizes of 100-300 μm and interconnective pore sizes of 30-100 μm. It was found that endochondral osteogenesis occurred in HA, while β-TCP was only intramembrane osteogenesis[19].
1.2.5 Effect of microstructure on vascularization
Previously, we mentioned the theory of “Cell- Material-Blood supply” interacting with trinity tissue regeneration. Blood supply acts as a fertilizer and is a key source of nutrients needed for cell and tissue growth. Without nutrition, the migration, proliferation, differentiation, and secretion functions of seeded cells can be negatively affected in which the cells in the implant can even die, failing to union with the host bone.
The timing and depth of blood vessel formation are closely related to the microstructure. Dense bioceramics were firstly used in the treatment of bone defects, but the clinical effect was not ideal. So, the porosity was designed to improve tissue repair ability[18]. The pores become the necessary three-dimensional space for cell habitation and tissue regeneration. In vivo, vascularization of porous β-TCP with the same interconnective pore size (120 μm) but four different macropore sizes (300-400, 400-500, 500-600, and 600-700 μm) was studied. The results showed that large diameter vessels rich in red blood cells formed in the β-TCP with large macroporous size. Although the number of blood vessels did not increase in the material with the size of 600-700 μm, the proportion of blood vessels with large diameters increased significantly. Vessels with a diameter >100 μm accounted for 25.9% of the total, and those with a diameter >200 μm accounted for 4.98%. Interestingly, in the group of 300-400 μm, only 14.7% of vessels with a diameter >100 μm were found. And no vessels with a diameter >200 μm were found in any group except the group of 600-700 μm. The volume of new blood vessels in the materials with macroporous size >400 μm was the highest, and there was no significant difference among the other three materials[31]. Other research has reached similar conclusions[32-33]. However, some scholars believe that the macroporous size has no significant effect on vascularization, and the interconnectivity between pores plays a key role in the formation of blood vessels[34]. Even if the macroporous size of the material is large with the lack of effective interconnection, blood vessels can only be on the surface and cannot enter the blind end and central area of the material. The new blood vessels cannot communicate with each other, resulting that it is difficult to form an effective vascular network.
To further analyze the mechanism and significance of interconnective pore size on vascularization, five kinds of pore size (70, 100, 120, 150, and 200 μm) β-TCP porous ceramics with the same macroporous size (300-400 μm) were evaluated in vivo[31]. We found that the vascularization process with a size ≤100 μm was relatively slow, and there was still no blood vessel growth in the center in 4 weeks. However, the material with a size >100 μm was completely vascularized and showed a peak of vascularization. And it was found that the lumen of new blood vessels passing through the interconnection became narrower, and larger after entering the hole, resembling a string-of-beads shape (Fig. 3). The number and diameter of new blood vessels of materials with the size of 150 and 200 μm were significantly better than those of the other three groups (70, 100, and 120 μm). The interconnection is the door for blood vessels to enter and leave the macropores, and plays a bottleneck role, determining the diameter and number of new blood vessels. Finally, we concluded that the porous materials with larger interconnective pore sizes can form more abundant and more evenly distributed blood vessels. However, materials with small pore sizes have an uneven distribution of blood vessels. New blood vessels are concentrated in the peripheral margin. It can be seen that the interconnective pore size plays an important role in the vascularization of the material. Other researchers have also demonstrated that internal connections play a key role in vascularization[6,34]. In terms of biological mechanisms, our team also investigated the interaction between interconnective pore size and endothelial cells[35]. The results suggest that the pore size can affect the expression of platelet endothelial cell adhesion molecule-1 (PECAM-1) and vascular endothelial growth factor (VEGF). Compared to materials with inner connection diameters of 100 and 120 μm, the content of NO in materials with an interconnective pore size of 150 μm was significantly increased. As the end product of the PI-3K/Akt/eNOS signaling pathway, NO plays a crucial role in promoting endothelial cell migration, proliferation, and survival during vascularization[75]. This revealed that the interconnective pore size of the material can affect the PI3K/Akt/eNOS signaling pathway for blood vessel growth. This is also consistent with the results of other research on the effect of microstructure on vascularization[36-37].
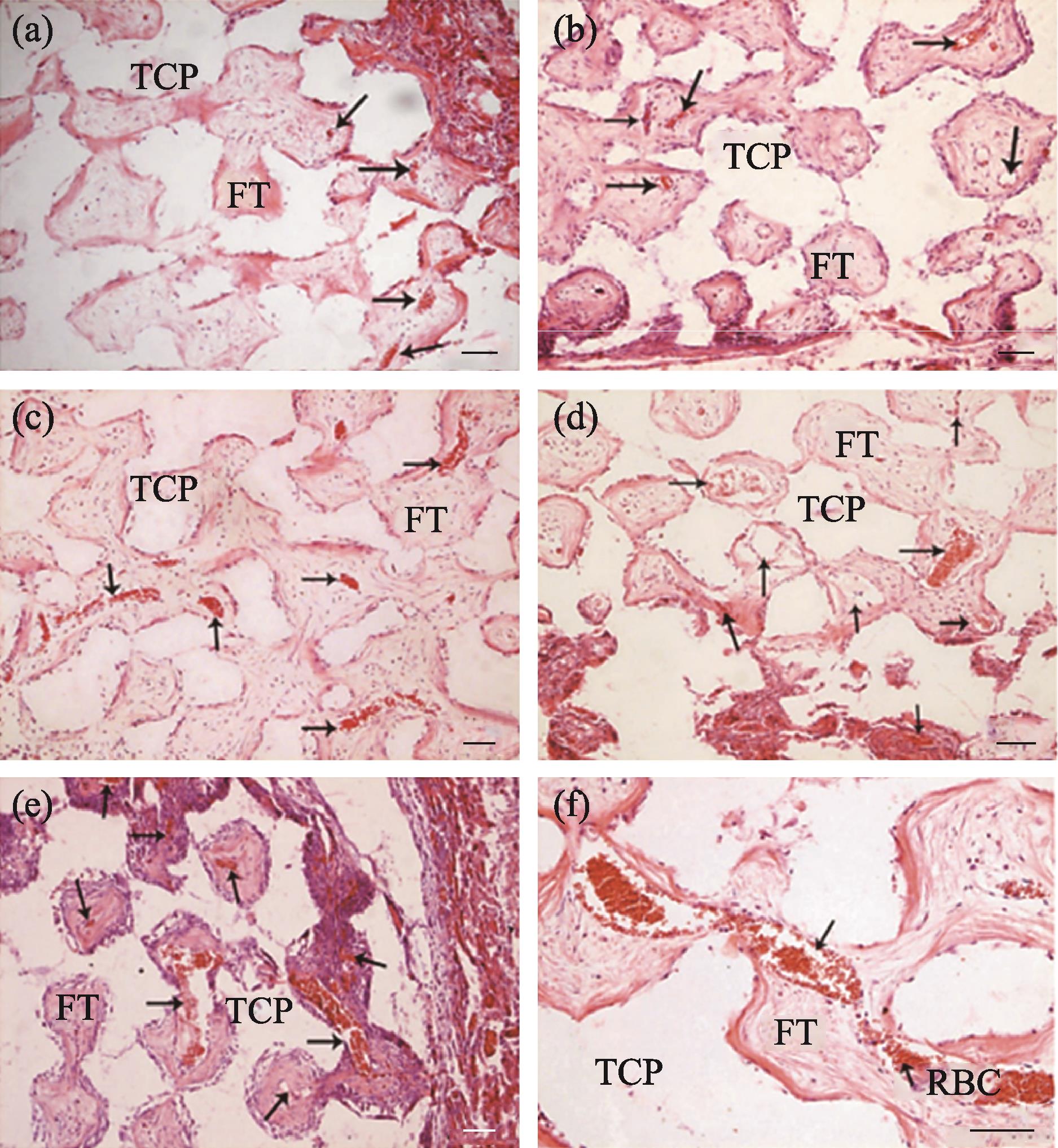
图 3.
Fig. 3. Different kinds of interconnective pore size β -TCP porous ceramics with the same macroporous size (300-400 μm) evaluated in vivo [31](a-e) Interconnective pore size is 70, 100, 120, 150, and 200 μm, respectively; (f) The lumen of new blood vessels passing through the interconnection became narrower and larger after entering the hole, resembling a string-of-beads shape. Scale bars in all images are 100 μm
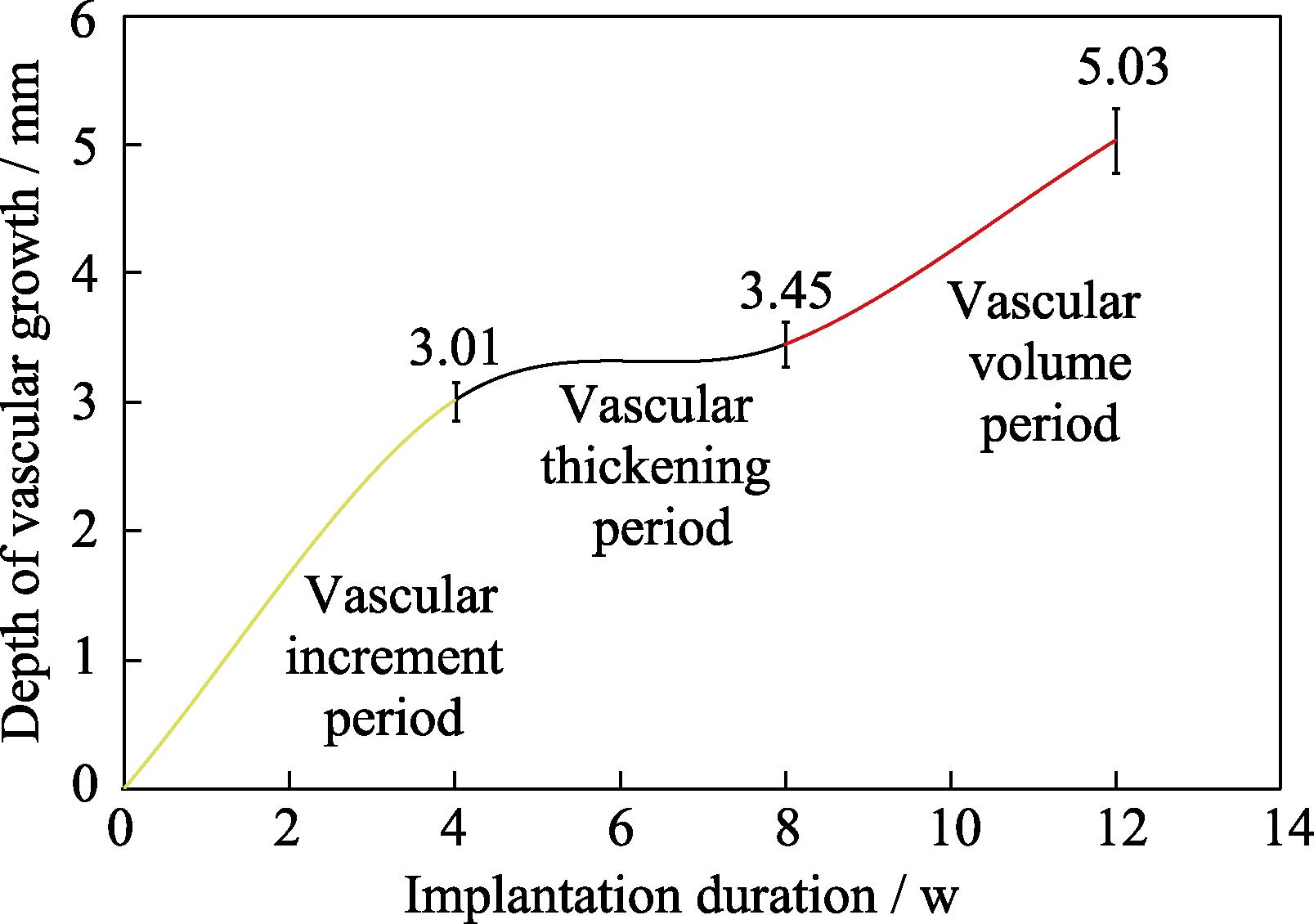
The early appearance of medical bioceramics transplantation is the same as hematoma and fiber mechanization due to trauma. The following changes may occur in the callus between the orthopedic graft and the broken end of the bone: vascular buds, brush vessels, dendritic vessels, string of beaded vessels, and spongy vessels. Then the capillary network is formed and anastomosed with each other, which grows into and diffuses deep into the material[17,38]. To further understand whether bioceramics can achieve the stealthy-growing of blood vessels with only unidirectional blood supply, one end of the cylindrical porous bioceramic rod was wrapped with titanium skin and implanted into the cancellous bone[17,39]. After 4 w repair, angiogenesis was evident around the implant. At 12 w, blood vessels were able to fully grow into the area shielded by the titanium skin, realizing a stealthing growth. The growth of blood vessels can be divided into three periods, namely, the period of vascular increment, vascular thickening, and vascular volume. The vascular growth rates of each period were 107.50 µm/d for the first 4 weeks after implantation, 15.71 µm/d for the second 4 weeks, and 56.43 µm/d for the last 4 weeks, averaging 59.90 μm/d (Fig.4). Other research about β-TCP unidirectional angiogenesis also demonstrated its performance in vaso-directed growth[40⇓-42]. Furthermore, these results suggest that blood vessels in suitable microstructures can be guided to grow to the ischemic region, which lays a theoretical basis for various vascularization treatments of bioceramics[43-44].
1.2.6 Structural adaptability suggestions
1) The downstream of the interconnection is the area of high adhesion and proliferation of cells.
2) The siphoning effect of macropores affects material cell recombination.
3) The degree of interconnective affects cell proliferation and osteogenesis.
4) The interconnectivity plays a decisive role in vascular growth.
5) The microstructure can induce cells physically.
6) Adaptability parameters: macropore size (≥500 μm), interconnective pore size (120 μm), porosity (65%).
2 Basics: degradative adaptability
2.1 Degradation mechanism of bioceramics in vivo
Biologically, achieving the balance between tissue regeneration and material degradation is the ideal goal of degradation adaptability. The process of calcium phosphate- based bioceramic degradation to bone repair and reconstruction is a process of biological transformation from inorganic material to organic bone tissue. There are three main ways of degradation in vivo: chemical dissolution, physical dissolution, and biodegradation[45⇓-47].
Chemical dissolution, the most important degradation way of bioceramics, is a process for decomposing materials to ions when body fluids contact with materials and enter the interior of materials through the porous structure for dissolution. This process causes the pH to drop around the implant, and the acidic environment helps it degrade. With the gradual loosening of the structure caused by dissolution of body fluids, the surface area of the material gradually expands. This continuous formation of new interfaces provides a favorable three-dimensional space for bone repair. Eventually, the bioceramics will be completely degraded by release of Ca2+ and PO43-. The degradation and release of them can increase the local ion concentration, which is beneficial to cell proliferation, differentiation and new bone formation. The unused Ca2+ and PO43- released from the chemical dissolution process can also be stored in the body, or be excreted through urine and feces.
Physical degradation is a process due to mechanical factors or fluid washout, which causes the material to fragment or disintegrate into smaller forms or particles. This kind of degradation has a great impact on microstructure of the material and leads to a decline in mechanical properties[46]. Overall, it promotes the subsequent degradation process. This is because the fine particles produced by the two ways of degradation mentioned above can induce further biodegradation processes involving a variety of cells.
As for biodegradation, there are three classes of cells involved in this process[46]. The first is the macrophages (polykaryons) which associate with inflammatory response immediately after implantation. Depending on the bioceramic particle size, macrophages can direct phagocytosis, called intracellular degradation, or digest them by releasing enzymes and/or niche pH decreasing, called extracellular degradation. The second is the gradual recruitment of osteoclasts (corresponding to physiological polykaryons). Similar to the absorption mechanism of the natural calcified matrix, osteoclasts can form an acidic environment in the implant site, leading to the dissociation of the strong binding of Ca2+ and PO43-[48]. The third is mesenchymal cells, such as endothelial cells, osteoblasts, fibroblasts, etc. They possess the ability to phagocytose particles. Nevertheless, they are also involved in the formation of fibrous capsules, which limit bone in-growth and material degradation[49⇓-51].
2.2 Effect of chemical composition and porous structure on degradation
The chemical composition of calcium phosphate- based bioceramics is the most important factor affecting its degradation[52-53]. It is well known that tricalcium phosphate (β-TCP) is much more degradable than hydroxyapatite (HA). Although HA has good biocompatibility, its solubility is very small and almost insoluble. At present, β-TCP is the main medical bioceramics used in clinical practice[54]. However, the effect of the chemical constituents released by degradation of bioceramics is bidirectional[55]. On the one hand, it can improve the bioceramic absorptive activity of osteoclasts. On the other hand, a deleterious Ca2+ release gradient is adverse for cells and tissue.
Interestingly, the porous structure affects the degradation process. The author’s team found that the degradation process of calcium phosphate cement (CPC) is a gradual reduction of material from the periphery to the center, which is similar to the crawling replacement of autologous bone graft[45⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-56]. It is accompanied by lots of granules produced by the phagocytosis of macrophages and multinucleated giant cells. The degradation process of porous bioceramic (β-TCP) is centric, that is, the peripheral and central degradation is synchronous. Those small beginnings can swiftly grow into the foundation for new bone formation. Other research has also reported central degradation and osteogenesis phenomenon of porous materials with the same or different degradable- osteogenic components[33,57].
2.3 Different sites responding to degradation and osteogenesis
At different implantation sites, the degradation rate is different due to differences in blood supply and fluid volume[58]. To evaluate the degradation and osteogenic response at the implant site, the author’s team implanted bioceramics in the femoral diaphysis and condyle of rabbits. The results showed that the degradation rate was medullary cavity > cancellous bone> cortical bone whereas the osteogenic response was the opposite. Cortical bone is the best, followed by cancellous bone, which is also confirmed in the following clinical research[59]. The medullary cavity was the worst. And even at 24 w follow-up, degradation was negatively correlated with osteogenic response. We considered that this may be related to the local mechanical stimulation. Furthermore, this is consistent with the anatomy of the bone - there is no bone mass in the medullary cavity.
Hence, cancellous bone is an ideal site for the evaluation of medical bioceramics due to its relatively balanced property of osteogenesis and biodegradation, as well as the bone encapsulation environment[60]. Cortical bone is more suitable for evaluating the material’s load-bearing properties and the interaction with the surrounding soft tissue[61]. The sensitive degradation and persistent foreign-body reaction of the medulla seem to be more suitable for the evaluation of biocompatibility[62].
2.4 Effect of packing method on degradation
A prospective study was initiated by the author’s team, to compare β-TCP granules and allograft bone granules for the treatment of bone defects in patients with bone cysts[63]. In the early stage, due to the lack of experience, some cases were treated with a dense packing method to fill the bone defect, just like the clinical usage of allograft bone particles. However, at 29 months follow-up, the degradation of bioceramics in these cases was very poor, and the worst cases even showed no sign of degradation. With the change of packing method to the loose, its degradation was significantly improved. Specifically, the degradation rate of β-TCP group with loose packing, was (85.83±17.63)%, which was higher than (79.04±21.53)% of the group of allograft bone. And the group of β-TCP implanted densely was just (51.57±35.06)%. For this result, we considered that the bioceramic itself is a highly brittle material, and the dense packing method will destroy the pore structure and interconnection structure that are originally conducive to degradation (Fig. 5).

图 5.
Fig. 5. Effect of packing method on degradation[63](a, d) Radiographs of cases with loose packing; (b, c) Radiographs of cases implanted with β -TCP granules by dense packing
In addition, the degradation of structural grafting, as reported by Dehoux et al., was significantly slower than that of granular grafting[64]. They used wedged or granular β-TCP for bone defect filling after high tibial osteotomy (HTO). The results indicated that the degradation of structural grafting is slow and partially compared to the complete and rapid degradation of granular grafting. Though osseointegration has been achieved at an early stage, several long-term clinical studies have failed to confirm traces of complete degradation of structural graft at 5 a and even 10 a[65-66]. However, there is also research in which complete or almost complete degradation was observed at 3.5 a[67]. In contrast, granular graft generally degrades completely within 1-2 a after surgery[63,68 -69]. It is not negligible that this is also correlated with the amount and volume of grafting. Although structural grafting degrades slowly, its intact structural shape has its unique advantages in terms of mechanical support and angle maintenance. If used at the weight-bearing site, it can assist the fixation device to a certain extent immediately, and make its long-term fixation more stable after osseointegration. Therefore, orthopedic surgeons are supposed to choose the appropriate grafting method according to the needs of clinical application.
2.5 Effect of age and complications on degradation
Age and complications are also related factors affecting the degradation. Bioceramics, implanted in patients younger than 20 years old, degrade significantly faster than those in older patients. This is consistent with the results of clinical research by our team and others[63,69]. The biological response to the changes in tissue
conditions after implantation in older patients may be less sensitive than that in the younger due to decreased cell mass, decreased angiogenic capacity, or impaired mechanoadaptation[70]. In addition, if postoperative complications occur, such as infection and tumor recurrence, the local microenvironment of the implanted tissue can be affected and the physicochemical degradation process of the material can also be adversely affected[63].
2.6 Degradative adaptability suggestions
1) The intergranular binding degree determines the degradation mode.
2) The microstructure affects liquid exchange, determining the degradation rate.
3) The blood supply at the implantation site affects the degradation rate.
4) The structural shape and implanting method affect the degradation rate.
5) Degradation rate is proportional to implant volume and inversely proportional to age.
6) Adaptability parameters: degradation rate should be slower than osteogenesis, remaining > 3 m.
3 Essential: mechanical adaptability
3.1 Effect of microstructures on mechanical strength
Dense bioceramics have good mechanical strength but cannot guide tissue regeneration, making the therapeutic effect of bone defects not ideal. Porous bioceramics enhance the ability to guide tissue regeneration but lose mechanical strength at some extent, resulting in limited clinical applications. Therefore, accurate fabrication of bioceramic microstructures and enhancing mechanical strength of porous materials, have become international challenges. Numerous researches show that the mechanical strength of porous materials is mainly related to porosity, macroporous size, and interconnectivity, but has little relationship with micropores[18,71]. Specifically, the greater the porosity of the interconnecting porous structure, the worse the mechanical strength. The author’s team tested the compressive strength of bioceramics with approximately 100% interconnectivity[17]. The results show that, under the condition of the same macroporous size, the larger the interconnective pore size, the higher the porosity and the lower the mechanical strength. With the same interconnective pore size, the larger the macroporous size, the lower the porosity, but the higher the mechanical strength.
3.2 Mechanical enhancement of porous bioceramics
To improve strength and toughness of bioceramics, scholars have developed various techniques, aiming to improve the mechanical properties of porous bioceramics to meet the application needs while maintaining their excellent bone repair properties. The techniques of porous structure design, composition modification, and polymer coating have positive effects on the mechanical properties of bioceramics[72-73]. For solving the problem of mechanical adaptability from a single biomaterial, our team, inspired by natural bone anatomy, prepared a bioceramic with a dense/porous integration structure (Chinese Patent CN100540071) by injection molding[72]. That is a kind of homogeneous isomer bioceramic composed of a single β-TCP component. And the porosity of dense and porous structures is 5%-10% and 70%-90%, respectively. Mechanically enhanced bioceramics prepared by this technique can be divided into three types according to the structural characteristics: central type, marginal type, and dispersed type. All of them realize a seamless connection between dense and porous interfaces, ensuring stable mechanical properties and effective clinical treatment (Fig. 6(a, c, d)). At the initial stage of implantation, the dense body (about 120 MPa) can exert strong mechanical support. The porous body (more than 10 MPa) guided tissue regeneration and was gradually replaced by new bone to play the role of biomechanical support. The dense body can also gradually degrade, and finally realize the permanent biomechanical support by regenerated bone tissue. The wedge-shaped implant for high tibial osteotomy (HTO) is a good clinical example of this technology (Fig. 6(b)). Its compressive strength can reach 62 MPa, which is 30 times more than that of the entire porous one, and can meet the application requirements of the stressed part. This kind of graded/gradient porous design is beneficial to mechanical adaptability. With technological development and cost reduction of manufacturing, it is expected to be widely used in the field of bone defect filling, implant fixation, etc[74].
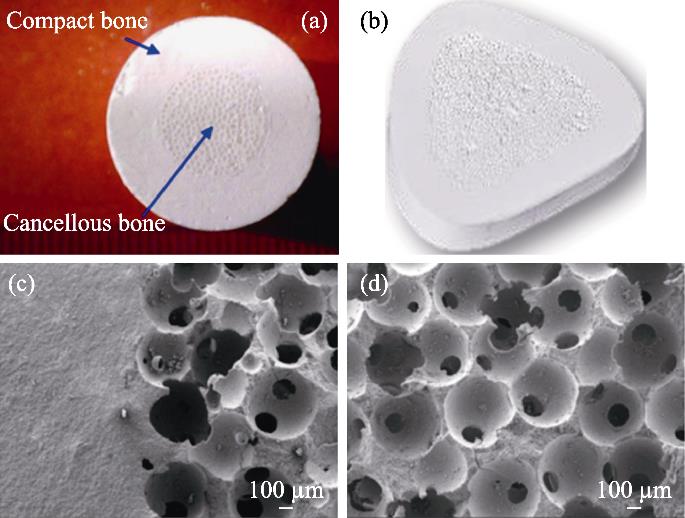
图 6.
Fig. 6. Mechanically enhanced bioceramics[72](a) Structure of mechanically enhanced bioceramics; (b) Wedge- shaped implant for high tibial osteotomy (HTO); (c, d) Microstructures of the bioinspired β -TCP bioceramics showing the dense/porous interface (c), and macroporous structure (d)
3.3 Suitable structure meeting requirements of biomechanical reconstruction
The subsequent biomechanical reconstruction is more important than immediate mechanical support after surgery. The causes of medical bioceramics failure can be roughly divided into 5 classifications[75-76]. Among them, mechanical failure includes soft-tissue failure, aseptic loosening and structural failure, which accounted for 12%, 19% and 17%, respectively. To avoid these problems, apart from mechanical enhancement techniques for bioceramics themselves, we can also consider mechanical adaptability through the combined use of multi-materials and multi-technologies. For instance, we designed a bird’s nest-like frame structure implant (Chinese Patent CN110882417B) that can be used with bioceramic granules packing to treat lacunar bone defects in the tibial plateau (Fig. 7). The bird’s nest frame can maintain the space structure of lacunar defects. Moreover, its grid structure can communicate inside and outside the frame, the bioceramic, and the bone tissue, and finally realize osseointegration to provide reliable biomechanical support. In addition, we also designed a long, hollow tubular frame structure implant that can also be packed with bioceramic granules (Fig. 8). In the early stage, it can immediately restore the length of the lower limb after tumor resection and provide reliable immediate
mechanical support. The internal packing of biomaterial can solve the problem of limited osseointegration of metal implants at the interface between host bone and implant[77-78]. It can also achieve internal osseointegration of the metal implant during long-term repair. Current literatures show that the probability of mechanical failure is lower for uncemented fixation than for cement, even if the difference is not significant[79]. So, we believe that this biological reconstruction implant design is more conducive to reducing the possibility of implant mechanical failure.
It is worth mentioning that the structural design of metal implants should avoid the phenomenon of stress shielding[80]. Due to the lack of mechanical stimulation, rigid internal fixation is not conducive to bone defect repair[81]. And this could eventually lead to the failure of the implant[82]. Using additive manufacturing technology to design porous structures was suggested to be an effective solution[83]. However, we have a failure case that can be used as a reference for negative design[77]. For tumors in the distal femur, we designed a metal implant that resembles a lowercase “d”. At 10 m follow-up, the implant broke due to stress concentration and insufficient support, at the joint between the metal body and the extended steel plate (Fig. 9). Therefore, these situations should be avoided in the selection of internal fixation devices and the structural design of medical bioceramics.
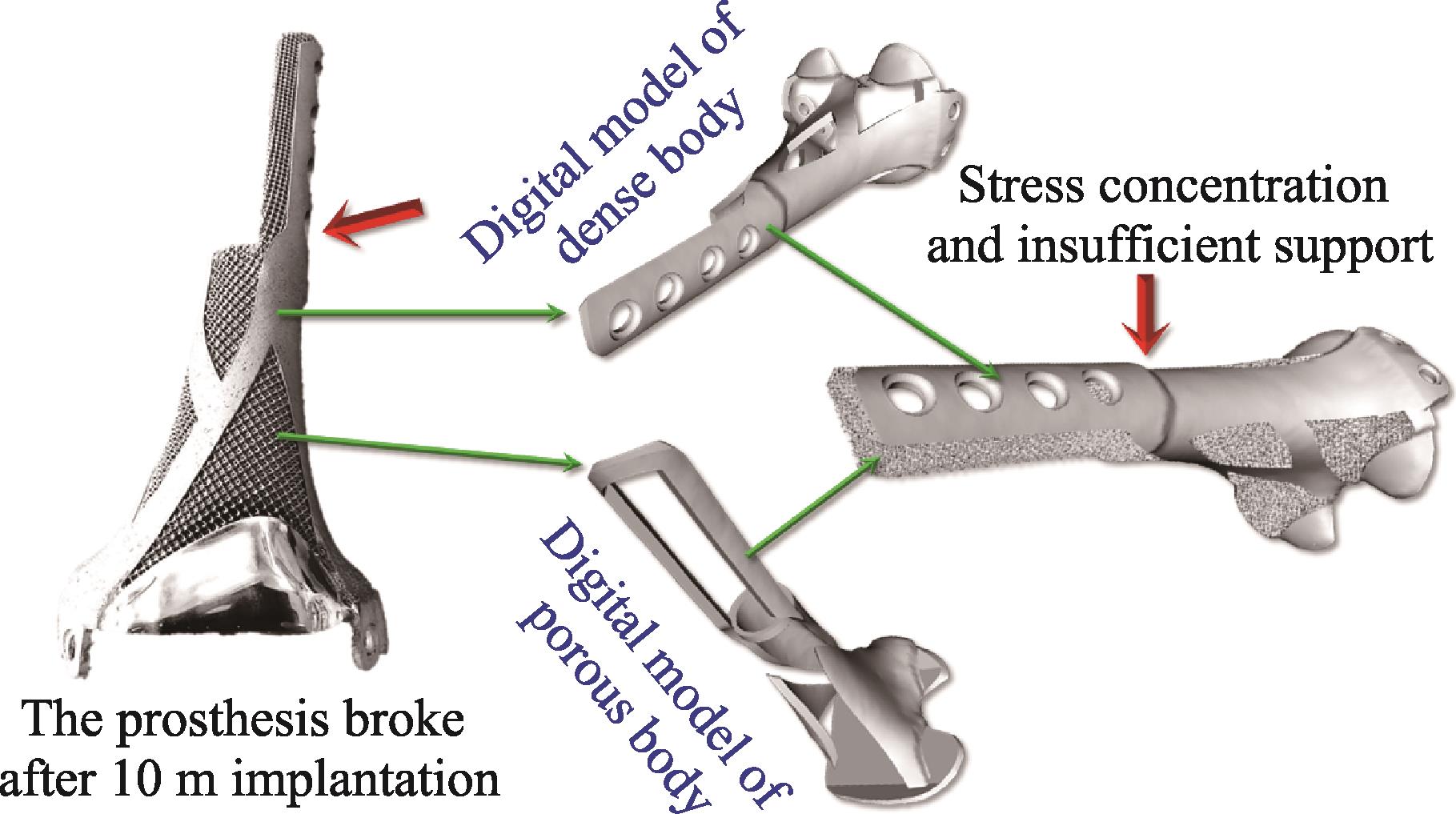
图 9.
Fig. 9. Failure clinical case showing the implant broken due to stress concentration and insufficient support
3.4 Mechanical adaptability suggestions
1) The compressive strength is proportional to the macroporous size and inversely proportional to the interconnection size.
2) Enhancement technology can expand the scope and prospect of clinical application.
3) Biomechanical reconstruction capacity is more important than initial mechanical strength.
4) Multiple materials and technologies can be combined to meet operation and regeneration needs.
5) Excessive internal fixation strength affects regeneration and biomechanical reconstruction.
6) Adaptability parameters: the mechanical strength must meet the requirements of clinical operation and treatment.
4 Assistance: clinical adaptability
4.1 Therapeutic techniques
Therapeutic techniques of medical bioceramics affect the prognosis. The most common method is direct implantation[59,63 -64]. To further improve clinical efficacy and application adaptability, scholars developed various therapeutic techniques. Firstly, bioceramics are used in combination with antibiotics to meet the clinical need for anti-infective treatment of various complex trauma, infection, and skeletal system diseases such as tumors. Yuan et al. [84] doped nanosized-Ag in β-TCP to obtain a composite bioceramic that can repair bone defects and seve as an anti-infection agent. They used β-TCP as carrier to construct the sustained-delivery system of rifampicin, which provided a new idea for the treatment of spinal tuberculosis[85]. Secondly, bioceramics are used in combination with seed cells to meet the needs of repair targets. Guo et al. [86-87] filled the osteochondral defect by bioceramics with chondrocytes/autologous mesenchymal stem cells, and the new cartilage tissue was completely covered after 24 w (Fig. 10). Li et al. [88] used β-TCP loaded with osteoblasts derived from bone marrow mesenchymal-stem-cell, for the treatment of critical-sized bone defects to enhance the bone repair effect. Several clinical research also demonstrated the beneficial effect of this surgical technique in repairing bone defects[89⇓⇓-92]. Thirdly, bioceramics are used in combination with platelet factors or growth factors to acquire specific tissue responses and optimal bioavailability. Lu et al. [93] combined β-TCP with platelet-rich plasma (PRP) for the treatment of femoral head necrosis after core decompression. This surgical technique can significantly relieve patients’ pain in short term, achieve better functional outcomes, and delay disease progression. Besides, a randomized, multicenter research showed that at 36 m follow-up, the lumbar fusion grade of the HA loaded with bone morphogenetic protein-2 (BMP-2), was higher than that of the auto-iliac bone graft. Adverse events such as tumorigenesis that were feared by growth factor use were not observed.
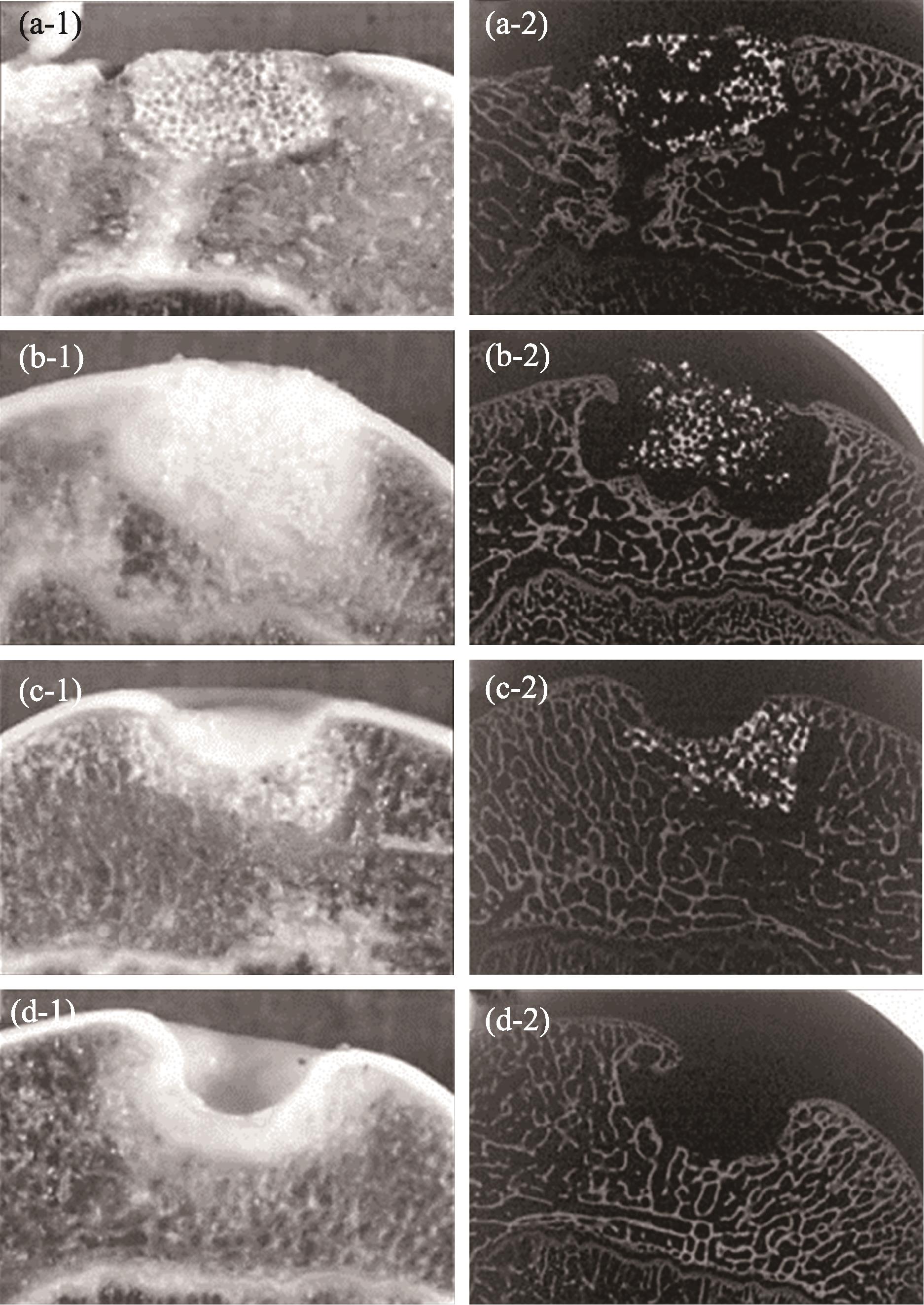
图 10.
Fig. 10. Bioceramics used in combination with chondrocytes to achieve better cartilage tissue repair[85](a) Repaired with bioceramic-chondrocyte constructs implant, 2 w postsurgery; (b) Repaired with bioceramic-chondrocyte constructs implant, 24 w postsurgery; (c) Repaired with bioceramic without cells implant, 24 w postsurgery; (d) Defect without any implant (control), 24 w postsurgery
4.2 Matching surgical auxiliary instruments
The author’s team has conducted research on the treatment of osteonecrosis of the femoral head (ONFH) by minimally invasive vascularization technique with a bioceramic rod[44]. The medical bioceramics used in this research were β-TCP, consisting of dense granules, porous granules, and porous rods. To cooperate with the performance of this surgical technique, the author’s team specially designed the “bioceramic rod minimally invasive treatment of ONFH instrument set”, which mainly includes: minimally invasive reamers (Chinese Patent CN102038544B) and multifunctional grafting tube (Fig. 11). This set of tools is simple and easy to operate. Specifically, because the neck of the femur is smaller than the femoral head, traditional tools cannot effectively clean up necrosis by debridement, especially ossified bone, from the head through the neck. According to the structural characteristics and anatomy size of the femoral head and neck, the author’s team designed minimally invasive reamers (Fig. 11(c)). In addition, the multifunctional grafting tube can safely and accurately implant β-TCP granules and rods into the necrosis sites. And it can be also used as a working channel for sequestrum clearance, material packing, and bioceramic rod implantation (Fig. 10(d, e)). Furthermore, the device can implant any material needed for treatment into the corresponding site under the blind vision.
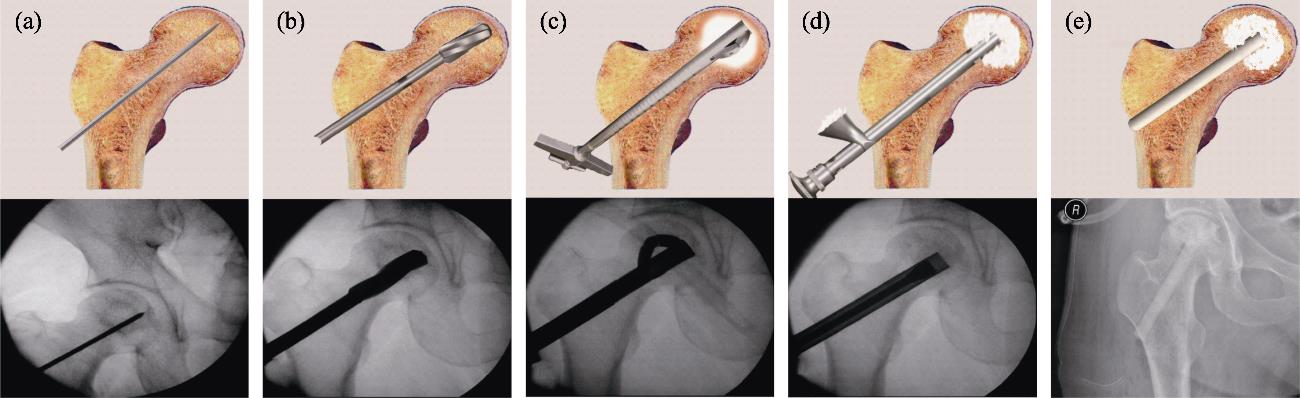
图 11.
Fig. 11. Standard surgical procedure for ONFH treatment, performed with surgical ancillary instruments[44](a) Insertion of the Kirschner wire under fluoroscopy; (b) Core decompression by drilling; (c) Necrosis debridement by minimal reamers; (d) Bioceramic granules packing; (e) Insertion of the porous bioceramic rods
4.3 Multi-materials, structures, and technologies to achieve clinical adaptability
Unlike animal models, the problem of bone defects encountered in clinical practice is complex and variable, especially for large volume and long segmental bone defects. According to the local environmental conditions and functional reconstruction needs, the application of multiple materials, structures, and technologies can complement each other and achieve clinical adaptation. For cases such as atrophic nonunion or chronic osteomyelitis, some studies have suggested the use of bioceramics combined with induced membrane technology. Furthermore, it can be also combined with autologous bone, growth factor (BMP-2 or BMP-7), or seed cells (autologous BMSCs)[94⇓⇓-97]. For cases such as HTO or ONFH, some researchers have suggested the use of multi-structures implants to meet specific functional adaptability needs. Tanaka et al.[67] designed two different porosity bioceramics with 60% and 75% respectively for HTO. The compressive strength of the former is approximately sevenfold greater than that of the latter, and it is used to assist the Puddu plate for mechanical support on the cortical side. The latter is used for the cancellous side. And the larger porosity facilitates bone in-growth and faster stabilization of interface bonding. Besides, the author’s team applied dense granules, porous granules, and porous rods of bioceramics to the treatment of femoral head necrosis at the same time. While the dense structure and rod-like structure meet the mechanical support needs of the femoral head, the porous structure can guide blood vessels and new bone to grow into the osteonecrosis area.
Last but not least, with the development of digital orthopedics represented by 3D printing technology, metal-personalized implants have entered clinical trials and achieved preliminary results[98⇓-100]. Although it has certain advantages, metal implants are not bioactive, and there is still a high risk of infection, fracture, loosening, and other complications in long-term evaluation. The main reason is that it is difficult to form a stable osseointegration between the internal surface of the implant and the host bone[101]. Therefore, the author’s team proposed the concept of “In vivo Bioreactors” (Chinese Patent CN104188738B), regarding the local physiological environment in the body as a reactor to achieve large-sized in situ reconstruction in the form of a complex scaffolds and biomaterials[102]. Guided by this concept, we designed the “In Vivo Bioreactor” for bone defect repair based on the 3D printed metal implant filled with porous bioceramic granules. The 3D-printed metal implants as basic components can meet the needs of mechanical continuity and support. The bioceramics can recruit growth factors and stem cells in situ, and guide blood vessel and tissue regeneration at the same time. Together, they achieved the goal of “In Vivo Construction”. The “In Vivo Bioreactor” was first evaluated in an animal model, and the histological results showed that it could effectively form a “Rebar Coagulated Bone” structure in which the metal implant was fused with the new bone to achieve a strong mechanical effect (Fig. 12)[102]. Then, we performed prospective clinical research[77]. Just as the typical case showed, we applied a multi-material, structural, and technical bone defect repair solution, using the 3D-printed implant, vascularized fibula, bioceramic granules, and metal plates (Fig. 13).
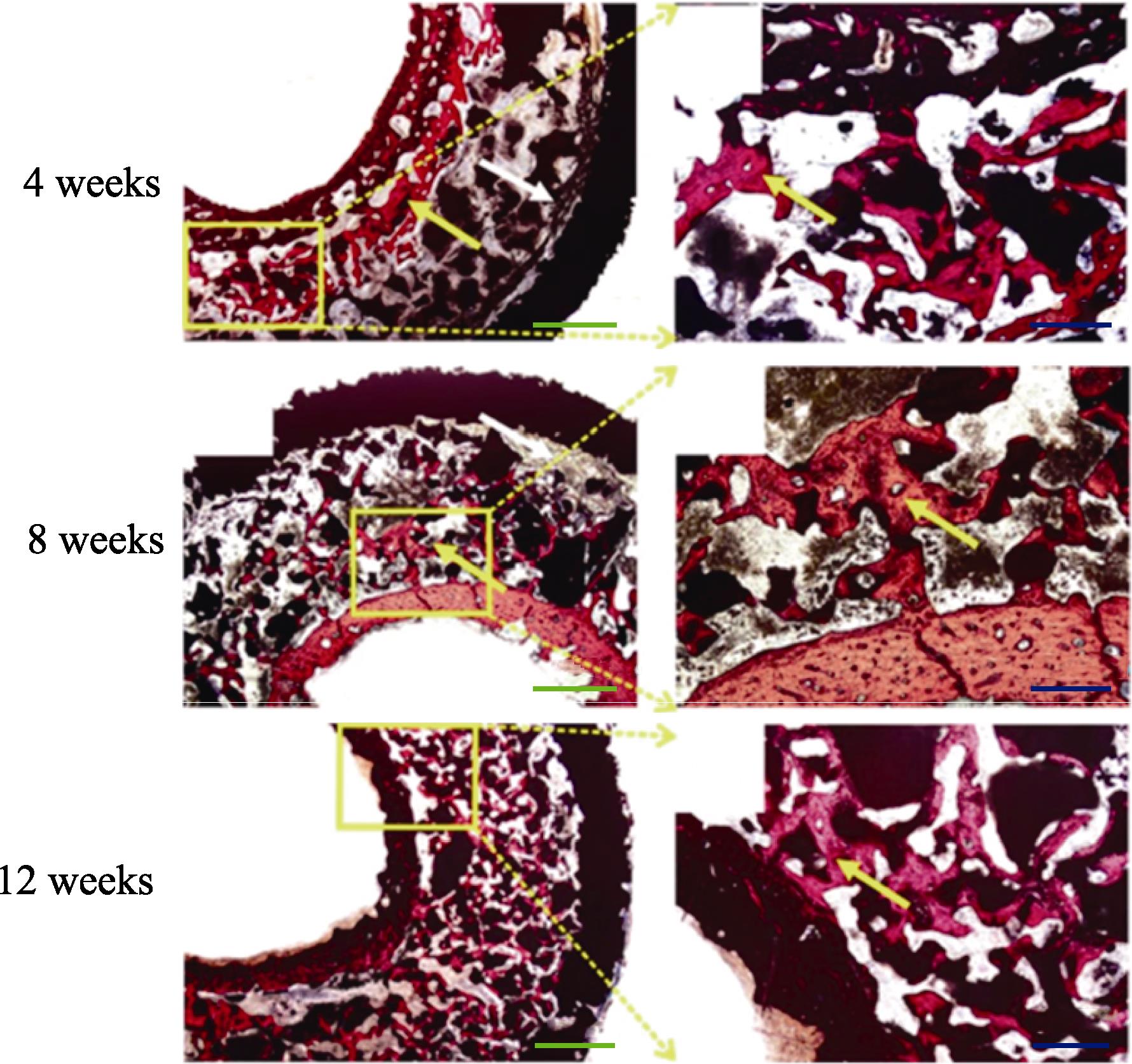
图 12.
Fig. 12. Van Gieson staining of “In Vivo Bioreactor” in rabbits showed the “Rebar Coagulated Bone” structure[102]Yellow arrow: Newly formed bone; White arrow: Connective. Scale bar: 50 μm (blue), 20 μm (green); Colorful figures are available on website
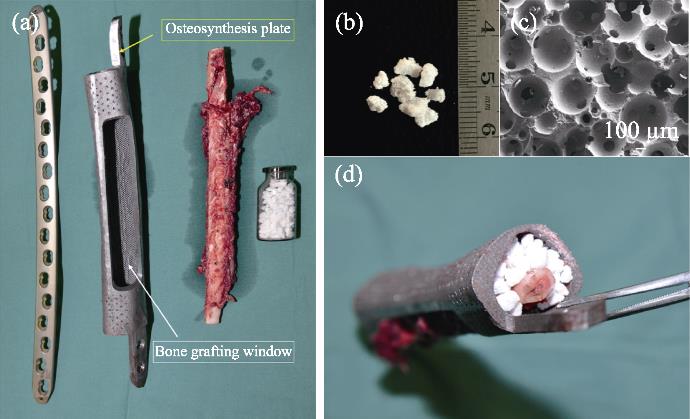
图 13.
Fig. 13. Application of“In vivo Bioreactor” in operation[77](a) Multi-material, structural, and technical bone defect repair solution; (b) Bioceramics granules being used; (c) Bioceramics microstructure; (d) Composite in operation
4.4 Clinical adaptability suggestions
1) Specialized auxiliary instruments affect treatment techniques and fusion of implants.
2) Therapeutic techniques affect the clinical effect of medical bioceramics.
3) The complementary function of multiple materials, structures, and technologies.
4) Therapeutic techniques are essential for realizing the value of medical bioceramics.
5) Treatments should be more precise, less invasive, and more effective.
6) Adaptability parameters: technologies, implants, auxiliary instruments into a trinity.
5 Summary
Orthopedic clinical requirements for dream orthopedic implants are rapid bone regeneration and long-term mechanical service capabilities. So how can bioadaptability materials be transformed into orthopedic implants that meet practical clinical needs? In this perspective, our team summarized the research and application experience of medical bioceramics (mainly calcium phosphate-based) over the years, further discussed our views from the aspects of structure, degradation, mechanics, and application around the theme of “functional bioadaptability”, and put forward suggestions on design, manufacture, and application. These recommendations should not be set in stone, and more “appropriate” parameters are bound to emerge as the manufacturing process progresses and research progresses. Therefore, functional bioadaptability should be a goal, and the achievement of this goal must be the result of interdisciplinary efforts among biologists, materials scientists, manufacturing engineers, and orthopedic surgeons.
[1] LEMONS J E. Ceramics: past, present, and future[J]. Bone, 1996: 121S.
[2] WILLIAMS D F. The plasticity of biocompatibility[J]. Biomaterials, 2023: 296: 122077.
[3] WILLIAMS D. Revisiting the definition of biocompatibility[J]. Med. Device Technol., 2003: 10.
[8] DIAO J, OUYANG J, DENG T, et al. 3D-plotted beta-tricalcium phosphate scaffolds with smaller pore sizes improve
[9] LI M, FU X, GAO H, et al. Regulation of an osteon-like concentric microgrooved surface on osteogenesis and osteoclastogenesis[J]. Biomaterials, 2019: 216: 119269.
[12] LIU X, ZHAO N, LIANG H, et al. Bone tissue engineering scaffolds with HUVECs/hBMSCs cocultured on 3D-printed composite bioactive ceramic scaffolds promoted osteogenesis/ angiogenesis[J]. J. Orthop. Translat., 2022: 37: 152.
[13] LU Q, DIAO J, WANG Y, et al. 3D printed pore morphology mediates bone marrow stem cell behaviors
[14] DIAO J J, DING H W, HUANG M Q, et al. Bone defect model dependent optimal pore sizes of 3D-plotted beta-tricalcium phosphate scaffolds for bone regeneration[J]. Small Methods, 2019: 11.
[15] SONG C, LIU L, DENG Z, et al. Research progress on the design and performance of porous titanium alloy bone implants[J]. J. Mater. Res. Technol., 2023: 23: 2626.
[21] XIE Y, HARDOUIN P, ZHU Z, et al. Three-dimensional flow perfusion culture system for stem cell proliferation inside the critical-size beta-tricalcium phosphate scaffold[J]. Tissue Eng., 2006: 3535.
[22] XIE Y, ZHU Z, TANG T, et al. Using perfusion bioreactor for mesenchymal stem cell proliferation in large tricalcium phosphate scaffold[J]. Chinese J. Orthop., 2006: 1633.
[23] FORRESTAL D P, ALLENBY M C, SIMPSON B, et al. Personalized volumetric tissue generation by enhancing multiscale mass transport through 3D printed scaffolds in perfused bioreactors[J]. Adv. Healthc. Mater., 2022.
[27] ZHANG Z, DU J, WEI Z, et al. Numerical simulation of dynamic seeding of mesenchymal stem cells in pore structure[J]. Comput. & Mathemat. Appl., 2020: 88.
[28] KUBOKI Y, JIN Q M, TAKITA H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis[J]. Bone and Joint Surg.-Am., 2001: 83A: S105.
[29] MAHAPATRA C, KUMAR P, PAUL M K, et al. Angiogenic stimulation strategies in bone tissue regeneration[J]. Tissue Cell, 2022: 79: 101908.
[33] SHEN M, LI Y, LU F, et al. Bioceramic scaffolds with triply periodic minimal surface architectures guide early-stage bone regeneration[J]. Bioact. Mater., 2023: 25: 374.
[35] XIAO X, WANG W, LIU D, et al. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes
[39] LU Y J, WANG Z, LU X, et al. Minimally invasive treatment for osteonecrosis of the femoral head in ARCO stage Ⅱ and Ⅲ with bioceramic system[J]. Chin. J. Repar. Reconst. Surgery, 2019: 1291.
[41] KUNISADA T, HASEI J, FUJIWARA T, et al. Radiographic and clinical assessment of unidirectional porous hydroxyapatite to treat benign bone tumors[J]. Sci. Rep., 2020: 10: 21578.
[43] IKUTA K, NISHIDA Y, OTA T, et al. A clinical trial of a unidirectional porous tricalcium phosphate filling for defects after resection of benign bone lesions: a prospective multicenter study[J]. Sci. Rep., 2022: 12: 16060.
[46] ZHANG Y, SHU T, WANG S, et al. The osteoinductivity of calcium phosphate-based biomaterials: a tight interaction with bone healing[J]. Front Bioeng. Biotechnol., 2022: 10: 911180.
[47] STASTNY P, SEDLACEK R, SUCHY T, et al. Structure degradation and strength changes of sintered calcium phosphate bone scaffolds with different phase structures during simulated biodegradation
[53] TAJVAR S, HADJIZADEH A, SAMANDARI S S. Scaffold degradation in bone tissue engineering: an overview[J]. Int. Biodeter. & Biodegr., 2023: 105599.
[54] BOHNER M, SANTONI B L G, DOBELIN N. Beta-tricalcium phosphate for bone substitution: synthesis and properties[J]. Acta Biomater., 2020: 113: 23.
[57] SIMON J L, ROY T D, PARSONS J R, et al. Engineered cellular response to scaffold architecture in a rabbit trephine defect[J]. J. Biomed. Mater. Res. A, 2003: 275.
[61] ZHI W, WANG X, SUN D, et al. Optimal regenerative repair of large segmental bone defect in a goat model with osteoinductive calcium phosphate bioceramic implants[J]. Bioact. Mater., 2022: 11: 240.
[67] TANAKA T, KUMAGAE Y, SAITO M, et al. Bone formation and resorption in patients after implantation of beta-tricalcium phosphate blocks with 60% and 75% porosity in opening-wedge high tibial osteotomy[J]. J. Biomed. Mater. Res. B Appl. Biomater., 2008: 453.
[68] OGOSE A, HOTTA T, KAWASHIMA H, et al. Comparison of hydroxyapatite and beta tricalcium phosphate as bone substitutes after excision of bone tumors[J]. J. Biomed. Mater. Res. B Appl. Biomater., 2005: 94.
[71] ZHANG Y, ZHANG Q, HE F, et al. Fabrication of cancellous- bone-mimicking
[74] MIAO X, SUN D. Graded/gradient porous biomaterials[J]. Materials, 2009: 26.
[77] LU Y, CHEN G, LONG Z, et al. Novel 3D-printed prosthetic composite for reconstruction of massive bone defects in lower extremities after malignant tumor resection[J]. J. Bone Oncol., 2019: 16: 100220.
[78] KELLY C N, WANG T, CROWLEY J, et al. High-strength, porous additively manufactured implants with optimized mechanical osseointegration[J]. Biomaterials, 2021: 279: 121206.
[81] POBLOTH A M, CHECA S, RAZI H, et al. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep[J]. Sci. Transl. Med., 2018: 8828.
[84] YUAN J, WANG B, HAN C, et al. Nanosized-Ag-doped porous beta-tricalcium phosphate for biological applications[J]. Mater. Sci. Eng. C Mater. Biol. Appl., 2020: 114: 111037.
[85]
[98] JUN F, ZHENG G, HONGBIN F, et al. Aplication of 3D-printed prosthesis on construction of long segmental bone defect after tumor resection[J]. Chin. J. Orthop., 2017: 433.
[100] BROWN T S, SALIB C G, ROSE P S, et al. Reconstruction of the hip after resection of periacetabular oncological lesions: a systematic review[J]. Bone Joint J.: 2018.
[101] THOMAS D, SINGH D. 3D-printing for engineering the next generation of artificial trabecular bone structures[J]. Int. J. Surg., 2017: 46: 195.
[102] GAO P, ZHANG H, LIU Y, et al. Beta-tricalcium phosphate granules improve osteogenesis
Article Outline
郑嘉乾, 卢霄, 鲁亚杰, 王迎军, 王臻, 卢建熙. 医用生物陶瓷的功能性生物适配机制及应用[J]. 无机材料学报, 2023, 39(1): 1. Jiaqian ZHENG, Xiao LU, Yajie LU, Yingjun WANG, Zhen WANG, Jianxi LU.