荧光导航冷冻聚焦离子束减薄技术的研究进展封底文章
Eukaryotic cells have numerous cellular structures, including a variety of organelles and macromolecular complexes. These structures have specific physiological functions and work interactively to perform certain cellular activities. Therefore, studying these structures in their native state is essential to understand the real physiological processes in the cells. In situ investigation of cellular structures does not only provide morphology, distribution, and abundance information, but also reveals their interaction mechanisms, thereby providing new insights into the understanding of life.
Cryo-electron tomography (cryo-ET) is currently the principal technique to resolve the in situ structures of biological specimens. By collecting tilted series of transmission electron images and performing image reconstruction, cryo-ET determines the 3D structures of bio-specimens with a nanometer-level resolution. A prerequisite for applying cryo-ET is to fix the sample under cryogenic conditions. High-pressure freezing and plunge freezing are well-established cryo-fixation methods that preserve biological specimens in their near-native state in vitreous ice. Benefiting from these techniques, cryo-ET has been widely applied to cells and tissues.
One limitation to cryo-ET is its restricted imaging depth, which is typically a few hundred nanometers owing to the confined penetration capabilities of electrons. Therefore, reducing the thickness of the samples to that of lamellae of approximately 200 nm is necessary before applying cryo-ET. Focused ion beam (FIB) milling has been recently employed to prepare lamellae of bio-specimens for cryo-ET. Compared to traditional ultramicrotomy, FIB milling avoids artifacts such as distortions, crevasses, and compression when fabricating the lamella. However, conventional FIB milling does not allow site-specific milling, because in a dual-beam FIB/SEM system, FIB or SEM image only illustrates the surface morphology of the sample and cannot provide more information to recognize and localize the underlying interest targets. When milling cells with FIB, cutting at an arbitrary position can only hit abundant cellular structures such as Golgi apparatus or mitochondria but cannot be used to prepare lamellae containing specific targets. This drawback hinders the application of FIB in cryo-ET.
The “blind” milling can be improved by correlative light and electron microscopy (CLEM). In CLEM, the targets of interest are fluorescently labelled and can be identified by fluorescence imaging. After registering the light and FIB images, fluorescence signal can be used to guide the FIB to mill at specific sites. Currently, various light imaging modalities have been adopted to navigate FIB fabrication, including widefield microscopy, confocal microscopy, and Airyscan. Moreover, two major working routines, that is, pipelined and integrated workflows, have been established to perform fluorescence-guided FIB milling. Therefore, it is important and necessary to summarize the existing techniques and discuss the advantages and limitations of different working routines to provide guidelines for researchers to choose the appropriate protocols.
This study reviews the essential techniques involved in fluorescence-guided cryo-FIB milling. First, plunge freezing is introduced. Plunge freezing is the most commonly used technique to vitrify cells. The key aspects to obtain good plunge-frozen specimens are discussed, including the choice of electron microscope grids and supporting films (Fig. 2), available commercial instruments (Fig. 3), and standard protocols.
Second, as a popular method to prepare lamellae of vitrified cells, FIB milling is discussed in several aspects: the working principle is introduced; the relevant instrumentations are summarized, including dual-beam FIB/SEM system (Fig. 4), cryostage and cryotransfer systems (Fig. 5), and Autogrid and sample holder (Fig. 6); and the milling of frozen cells is outlined (Fig. 7).
Third, the principle (Fig. 8) and workflow (Fig. 9) of fluorescence-guided FIB milling is introduced. Pipelined and integrated workflows are described, and relevant commercial instruments are overviewed (Figs. 10 and 11). The different workflows and various systems are compared (Table. 1). The most recent developments of integrated solutions are discussed in detail. Sun Fei's research group and Ji Wei's research group from the Institute of Biophysics, Chinese Academy of Sciences have developed novel integrated light, ion, and electron microscopies (Figs. 12 and 13), thereby providing new avenues for performing accurate and efficient FIB milling at specific sites under fluorescence guidance.
In situ investigation of cellular structures using cryo-ET has recently become an interesting research topic. Fluorescence-guided FIB milling has been applied to mill vitrified biological samples at specific sites. The recent developments in integrated cryo-FLM-FIB/SEM systems and workflows provide efficient and accurate methods to fabricate cell lamellae containing desired targets. These innovations have the potential to serve as all-in-one solutions for site-specific cryo-lamella preparation for cryo-ET in the future.
1 引言
真核细胞内部有着十分丰富的超微结构,包括各种细胞器(内质网、高尔基体、溶酶体、线粒体、中心体等)以及各类生物大分子复合体(蛋白酶体、核糖体、核小体等)。这些超微结构具有精细的分工和特定的功能,但又不是孤立工作的,而是通过交互作用紧密协作以实现某项特定的生理功能。将超微结构单独提纯出来的传统研究方法已经无法满足最新的科研需要。只有在细胞原位获取的形态、丰度、分布和相互作用等信息,才能客观完整地反映出真实的生理过程,因此细胞原位结构解析是目前的一大研究热点。
冷冻电子断层扫描成像技术(cryo-ET)是目前原位结构解析最前沿的技术[1-2]。该技术的理论基础是1968年由De Rosier和Klug[3]提出的三维重构原理,即通过对同一区域进行多角度的透射电子成像后,再反向计算便可重构出成像对象的三维信息。这个理论将透射电镜成像从二维时代带入了三维时代。20世纪80年代,Dubochet等[4]发展了快速冷冻技术,将含水生物样品在近乎天然的生理状态下冷冻固定在非晶冰中[5-6],解决了传统电镜样品制备方法脱水失活的问题。高保真的样品制备和高分辨的三维解析开启了利用cryo-ET解析细胞原位结构的时代[7-9],极大地加深了人们在细胞层面对生命的认知。
cryo-ET的一个局限是其只能观察厚度在几百纳米以下的样品[10],因为主流300 kV透射电镜的电子束只能穿透300 nm左右的冷冻样品[11]。有限的成像深度在很大程度上限制了cryo-ET的应用范围,因为真核细胞的厚度有几个微米,组织样品的厚度达到百微米甚至毫米级别,这些样品都无法直接利用cryo-ET进行观察。要用cryo-ET对生物样品进行成像,首先要将样品减薄成200 nm左右的薄片。
冷冻聚焦离子束(cryo-FIB)切割是近年来发展出的一项冷冻生物样品减薄技术[12]。该技术使用高能粒子(镓、氦、氙、氖、氧、氩离子等)轰击样品表面,移除部分物质以实现减薄[13]。相较于传统的超薄切片技术,cryo-FIB可以有效避免机械切割过程中的样品扭曲、崩裂、变形等问题[14-15],适用于微小细胞切片的制备,逐渐成为cryo-ET样品减薄的主流方法[16-17]。
cryo-FIB切割的一个先天缺陷是无法对细胞内部的特定目标进行定点减薄。聚焦离子束(FIB)或扫描电镜(SEM)成像只能勾勒出细胞浅表的形貌信息,无法看到细胞内部的情况,因此难以对特定细胞结构进行识别和定位。利用FIB在细胞任意位置上进行盲切的传统方法只能大概率命中丰度较高的细胞器(如高尔基体、线粒体等),而对于稀疏的细胞结构(如线粒体等),FIB盲切几乎不可能准确命中[18]。因此,精准制备含有特定目标的冷冻生物样品切片成为cryo-FIB技术的一个难题,也成为影响cryo-ET应用范围的一个重要因素。
光电融合成像技术(CLEM)[19-22]可以弥补电镜成像无法特异性识别研究目标的缺陷。CLEM利用荧光成像技术(FLM)来表征特定的研究对象,再通过光电图像的配准,确定研究对象在电镜图像上的位置,进而辅助电镜数据的筛选和分析,已被广泛应用到透射电镜[23-27]和扫描电镜[28-29]的原位结构解析研究中。此外,CLEM也被用来引导FIB的定点切割[30],通过光镜和FIB图像的关联配准,可在FIB图像上定位研究目标,从而实现“有的放矢”的定点减薄,解决了传统FIB“盲切”的问题,拓展了cryo-ET的应用范围。本文围绕冷冻细胞的荧光导航FIB减薄,对相关的技术方法、仪器设备和工作流程进行了梳理,旨在帮助研究者选择出合适的样品减薄方案,精准高效地制备含有研究目标的冷冻细胞切片,提高cryo-ET的效率和成功率。
2 荧光导航FIB减薄相关核心技术
2.1 细胞快速冷冻技术
2.1.1 概述
电镜(EM)的成像环境为高真空,为了对含水生物样品进行电镜观察,首先将样品固定在真空兼容的状态中。传统的电镜样品制备方法包括染色、化学固定、脱水、树脂包埋、切片等一系列操作[31],这种基于化学固定的方法会引入不同程度的假象,影响细胞超微结构解析的真实性。为了解决化学固定造成的样品失真问题,人们尝试利用冷冻的方法固定生物样品。含水样品的冷冻固定必须避免晶体冰的形成,因为晶体冰不仅会刺破样品的微观结构,还会形成絮状衬度,影响透射电镜(TEM)的观察。为此人们发明了高压冷冻[32]和快速冷冻两种方法,分别对较厚的组织样品(厚度在500 μm以内)和较薄的细胞、生物大分子样品(厚度在20 μm以内)进行冷冻固定[33],可将生物样品在近乎天然的生理状态下固定在非晶态冰中[34](
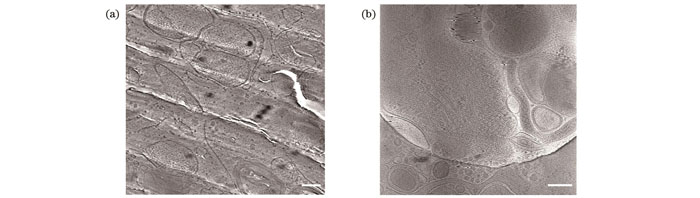
图 1. 冷冻固定在非晶冰中的生物样品(标尺:200 nm)。(a)利用高压冷冻固定的U2OS细胞超薄切片的透射电镜图像;(b)利用快速冷冻固定的PC12细胞的透射电镜图像
Fig. 1. Biological samples cryo-fixed in vitreous ice (scalebar: 200 nm). (a) TEM image of lamella of U2OS cell vitrified using high pressure freezing; (b) TEM image of PC12 cell vitrified using plunge freezing
2.1.2 载网和支撑膜
快速冷冻细胞样品的第一步是选择合适的载网和支撑膜。载网的材质通常为铜、钼、金等金属。固定细胞悬浮液一般会选择铜、钼等硬度较高的载网,避免冻样和转移过程中载网变形导致的样品破损。如果在载网上直接培养细胞,则选择毒性较小的金网。
载网上需覆盖一层有机支撑膜。支撑膜可以填补金属梁间的空隙,起到承托细胞的作用,通常选择带有孔洞的膜,方便冻样时从载网反面吸走多余的液体。孔洞可以为规则排列的形状,如商业化的Quantifoil[
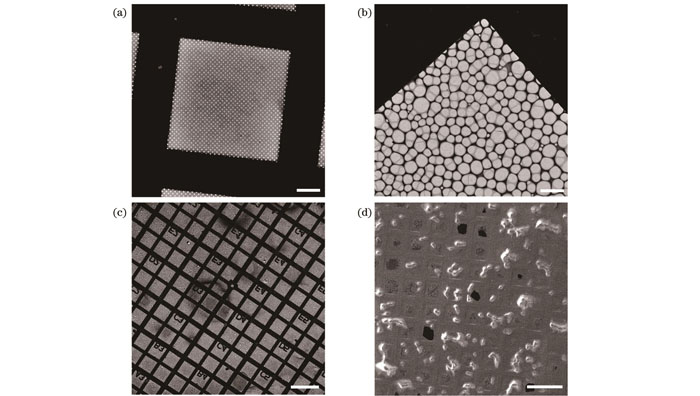
图 2. 用于细胞快速冷冻的电镜载网。(a)具有规则圆孔的Quantifoil载网(标尺:20 μm);(b)具有不规则孔洞的微栅载网(标尺:10 μm);(c)带有坐标的微栅载网(标尺:200 μm);(d)承载HeLa细胞的微栅载网(标尺:200 μm)
Fig. 2. EM grids for plunge freezing of cells. (a) Quantifoil grid with regular circular holes (scalebar: 200 μm); (b) lattice grid with irregular holes (scalebar: 200 μm); (c) lattice grid with coordinate markers (scalebar: 200 μm); (d) lattice grid containing HeLa cells (scalebar: 200 μm)
载网的目数以每孔容纳若干细胞为宜[
2.1.3 快速冷冻设备
快速冷冻设备在20世纪80年代由实验室开发[36-39],经过几十年的发展,已形成众多成熟的商业化产品(
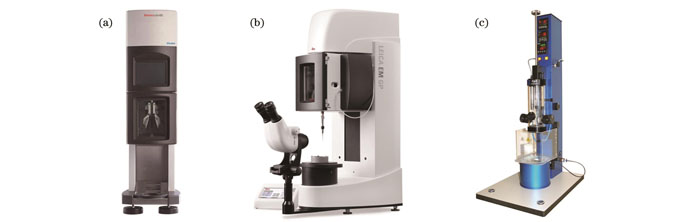
图 3. 商业化快速冷冻设备。(a)赛默飞公司的Vitrobot;(b)Leica公司的EM GP;(c)Gatan公司的Cp3
Fig. 3. Commercial instruments for plunge freezing. (a) Vitrobot from ThermoFisher; (b) EM GP from Leica; (c) Cp3 from Gatan
2.1.4 快速冷冻工作流程
快速冷冻的工作流程主要包括制冷剂制备、快速冷冻、样品回收等几个核心步骤[40-41]。即便商业化设备已经提供了十分完善的自动或半自动程序,在制样过程中仍有许多参数和条件需要用户根据具体的样品进行优化。对于细胞样品,快速冷冻的工作流程和注意要点如下。
1)将快速冷冻设备的部件(如泡沫盒、液态乙烷杯、镊子等)充分吹干,避免上一个用户使用之后的水蒸气残留。
2)更换滤纸,设置冻样设备的湿度及温度,对于细胞样品,温度一般设置在37 ℃左右,湿度在85%左右。
3)将设备空载运行一次,以免正式冻样时设备故障损坏镊子,浪费样品。
4)将冻样部件和样品盒完全浸入液氮中进行预冷。
5)对载网进行辉光放电(对于生长在载网上的细胞样品,跳过此步骤)。
6)制备液态乙烷。制备过程中观察乙烷形态,若能看到稳定的乙烷冰霜或者液态乙烷已部分变成固态,此时液态乙烷达到最低凝固点温度。
7)用镊子夹取载网,并将镊子卡入冻样设备。
8)使用移液枪吸取3~5 μL细胞悬浮液,加在载网有碳膜一侧。(对于生长在载网上的细胞样品,加磷酸缓冲盐溶液(PBS)以冲洗掉细胞培养液,避免“冻不透”。)
9)使用滤纸进行自动吸干,吸液时长一般为3~5 s。
10)将镊子及载网投入到液态乙烷中,完成快速冷冻。
11)将载网转移至样品盒。转移过程中保持载网不暴露在空气中。
12)将样品盒快速放进盛有液氮的50 mL离心管中,并将离心管转移至液氮罐中保存。
2.2 cryo-FIB减薄技术
2.2.1 概述
FIB切割使用的离子源主要包括液态金属离子源[42](例如镓等)和等离子体源[43](例如氩、氙等)。目前冷冻细胞样品减薄中使用的主要是液态金属镓离子源。镓具有低熔点、易挥发、低蒸气压的特点[44],在室温下以液态形式存在,在外加电压的作用下可电离出镓离子,再经过加速和一系列静电透镜的聚焦形成聚焦离子束。离子束的加速电压通常为5~50 keV,束流一般为几十pA到几nA,可产生5~500 nm直径的离子束,因此FIB十分适合在纳米尺度上进行细胞的加工。当FIB轰击样品表面时会移除焦点处的原子,再通过三维空间的扫描即可移除特定的体积,达到切割的效果。和SEM原理类似,FIB在扫描样品的过程中也会激发二次电子,同样可以形成二次电子图像。
FIB切割技术最早被应用在材料科学中,用于制备透射电镜薄片样品[45]或者加工固体材料[46]。不同于传统的固体材料,冷冻生物样品的加工必须在-135 ℃以下进行,否则非晶冰就会发生去玻璃化形成晶态冰[47]。2006年,Marko等[48]率先将FIB应用在非晶冰的切割中,证实了FIB不会引起非晶冰的去玻璃化。2007年,Marko等[12]进一步将这项技术应用在冷冻细菌的减薄中,获得了适用于透射电镜观察的细菌薄片样品,并验证了FIB切割不会引入影响透射电镜观察的缺陷。后来,Rigort等[49]完善了这项技术,针对cryo-ET建立了冷冻细胞FIB减薄的工作流程,并提出了利用CLEM引导FIB减薄的方案[50],使FIB成为cryo-ET样品减薄的主流方法。
2.2.2 cryo-FIB减薄设备
FIB通常被安装在SEM中使用,再配以气体注入系统(GIS)、冷台系统及多种二次电子、背散射电子探测器,形成功能强大的cryo-FIB减薄系统。目前主流SEM厂商均针对冷冻生物样品减薄提供了成熟的双束电镜(

图 4. 商业化双束电镜。(a)蔡司公司的Crossbeam双束电镜;(b)赛默飞公司的Aquilos双束电镜;(c)泰斯肯公司的Amber双束电镜
Fig. 4. Commercial dual-beam FIB/SEMs. (a) Crossbeam dual-beam FIB/SEM from Zeiss; (b) Aquilos dual-beam FIB/SEM from ThermoFischer; (c) Amber dual-beam FIB/SEM from Tescan

图 5. Quorum公司的冷台系统。(a)冷台模块;(b)冷阱模块;(c)带有磁控溅射镀膜功能的冷冻样品传输模块
Fig. 5. Cryostage system from Quorum. (a) Cryostage module; (b) cryotrap module; (c) cryogenic sample transfer module equipped with magnetron sputter coating function
2.2.3 cryo-FIB减薄流程
cryo-FIB减薄生物样品的工作流程已经发展得较为完善和成熟[51-52]。通常先将承载冷冻细胞的电镜载网(特别是柔软的金网)固定在Autogrid卡环中[
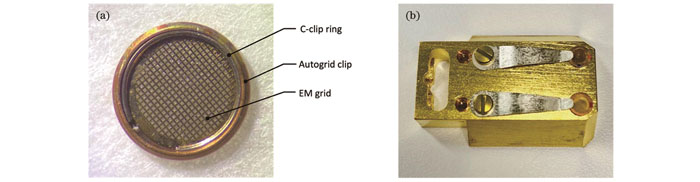
图 6. Autogrid及样品托。(a)装载在Autogrid中的电镜载网;(b)承载Autogrid样品的冷台样品托
Fig. 6. Autogrid and sample holder. (a) EM grid mounted in Autogrid; (b) cryostage sample holder containing Autogrid samples
目前最常用的减薄策略是在预设切片位置的上下方切削掉两个长方体以获得切片[
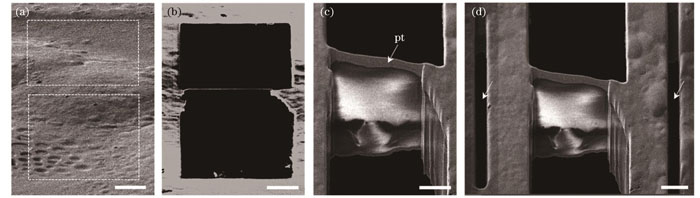
图 7. 冷冻细胞的FIB减薄(标尺:5 μm)。(a)冷冻在载网上的HeLa细胞的FIB图像,虚线框所示为准备切掉的部分;(b)减薄后细胞切片的FIB图像;(c)减薄后细胞切片的SEM图像;(d)细胞切片两侧微伸缩缝(箭头)的SEM图像
Fig. 7. FIB milling of vitrified cells (scalebar: 5 μm). (a) FIB image of HeLa cells vitrified on grid with part to be cut off shown in dashed box; (b) FIB image of cell lamella after milling; (c) SEM image of cell lamella after milling; (d) SEM image of cell lamella with micro-expansion joints (arrows)
对于cryo-ET,切片的厚度通常控制在300 nm以内,样品越薄,电子通透性越好,得到的图像衬度和分辨率越高[51]。切片的宽度通常为10~20 μm,由于样品内部应力的存在,越宽的切片面临的弯曲、褶皱和破损的几率也就越大。为了释放应力,在减薄前在切片两侧加工两条微伸缩缝(micro-expansion joints)[
2.3 荧光导航cryo-FIB减薄技术
2.3.1 概述
作为CLEM的一种应用形式,荧光导航cryo-FIB减薄的原理与CLEM类似:首先对荧光标记的细胞分别进行冷冻光镜[55]和cryo-FIB成像,再将光电图像进行关联匹配,根据荧光信号在FIB图像中的位置识别和定位研究目标,由此确定FIB的切割位点(
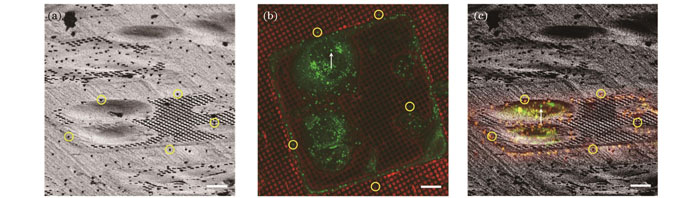
图 8. 荧光导航cryo-FIB减薄原理(标尺:10 μm)。(a)HepG2细胞的FIB图像,圆圈标注出5个用来配准的荧光珠;(b)图8(a)的光镜图像,圆圈标注图8(a)中对应的5个荧光珠,箭头标注选取的研究目标;(c)FIB和光镜的配准图像,箭头指示图8(b)中选取的研究目标
Fig. 8. Principle of fluorescence-guided cryo-FIB milling (scalebar: 10 μm). (a) FIB image of HepG2 cells with five fluorescent beads selected for image registration shown by circles; (b) light microscopy image of Fig. 8(a) with five fluorescent beads in Fig. 8(a) shown by circles and selected interest target shown by arrow; (c) superimposition of FIB and light microscopy images after image registration with target chosen in Fig. 8(b) shown by arrow
荧光导航cryo-FIB减薄目前有两种方案:分体式方案[50,59,61-62]和集成式方案[56-58,60]。在分体式方案[
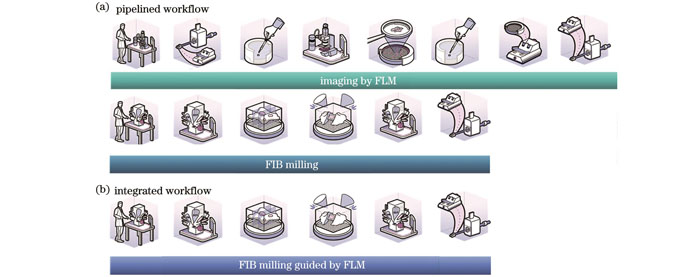
图 9. 荧光导航cryo-FIB减薄方案(图片来自www.nanoscience.com)。(a)分体式方案;(b)集成式方案
Fig. 9. Schemes of fluorescence-guided cryo-FIB milling (image source: www.nanoscience.com). (a) Pipelined scheme; (b) integrated scheme
表 1. 不同荧光导航FIB减薄策略的比较
Table 1. Comparison of different fluorescence-guided FIB milling strategies
|
2.3.2 分体式荧光导航cryo-FIB减薄设备与工作流程
分体式荧光导航FIB减薄设备主要由独立的冷冻光学显微镜和cryo-FIB/SEM系统构成。cryo-FIB/SEM在前文已介绍过,此处介绍冷冻光学显微镜。冷冻光学显微镜系统主要由光学低温恒温器和荧光显微镜两部分组成。光学低温恒温器通常是一个带有光学窗口的隔热腔室,利用绝热材料或者真空进行隔热,利用循环或者静态的液氮对隔热腔室内的样品进行制冷。利用显微物镜的光学窗口观察低温下的样品,实现低温光学成像。光学低温恒温器既有实验室开发的原型机[19,23,26,50,63-67],也有丰富的商业化产品[
图 10. 商业化光学低温恒温器和cryo-FLM成像系统。(a)Instec公司的HCS621GXY低温恒温器;(b)Linkam公司的CMS196低温恒温器;(c)Oxford Instruments公司的MicrostatN低温恒温器;(d)Janis公司的ST500低温恒温器;(e)搭载Linkam低温恒温器的蔡司冷冻共聚焦成像系统;(f)搭载自研发低温恒温器的Leica冷冻成像系统
Fig. 10. Commercial optical cryostat and imaging systems by cryo-FLM. (a) HCS621GXY Cryostat from Instec; (b) CMS196 cryostat from Linkam; (c) MicrostatN cryostat from Oxford Instruments; (d) ST500 cryostat from Janis; (e) Zeiss confocal cryo-imaging system equipped with Linkam cryostat; (f) Leica cryo-imaging system equipped with self-developed cryostat
分体式荧光导航cryo-FIB减薄工作流程如
分体式工作流程的另一缺点是样品需要多次转移。一般来讲,冷冻光学显微镜和cryo-FIB/SEM使用的样品托和传输系统均不相同,冷冻样品在跨系统传输中需要更换样品托,再分别用不同的传输机构进行换样操作。多次的样品转移不仅增加了操作难度和工作量,还容易造成样品的冰污染、升温、变形甚至破裂[13]。此外,减薄完成后,如果想对切片再次进行荧光检查,就需要将样品从cryo-FIB/SEM中传出,更换样品托后再传入冷冻光学显微镜中拍摄。重复的样品转移意味着切片受损的风险也会加倍,为了保护来之不易的脆弱切片,人们通常不愿意再次检查荧光,而是将切片直接传入到透射电镜中进行检查,这就进一步降低了透射电镜的成像效率。
针对分体式工作流程的这些问题,人们也在不断开发新的配件和方法以提高其效率和成功率。为了提升光电图像配准算法的易用性,Arnold等[59]将他们的配准软件设置为开源,方便科研人员使用。为了减少样品的转移次数,Kuba等[18]开发了可兼容冷冻光学显微镜和cryo-FIB/SEM的样品托,样品在进行冷冻光学显微镜成像后可直接送入到cryo-FIB/SEM中进行关联减薄,在一定程度上降低了样品的损坏几率。
2.3.3 集成式荧光导航cryo-FIB减薄设备与工作流程
近年来,人们尝试将冷冻光学显微镜与cryo-FIB/SEM系统进行集成。2019年,Gorelick等[56]将宽场荧光显微镜整合到商业化双束电镜中,获得了第一套集成式光电联用双束电镜。这套系统使用发光二极管(LED)作为照明和激发光源,将物镜安装在电镜腔室内部,将光学部件挂在电镜外壁上,实现了同一真空腔室内的荧光导航FIB减薄。与这套光学系统的结构类似,Delmic公司在2021年推出了商业化宽场荧光成像模块METEOR[57][
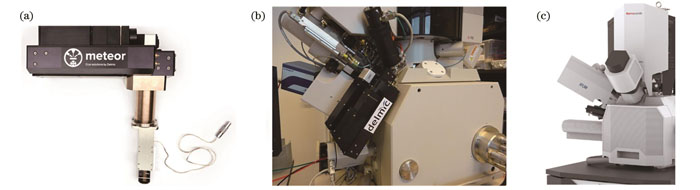
图 11. 商业化集成式cryo-FLM-FIB/SEM系统。(a)Delmic公司可适配双束电镜的宽场荧光成像模块METEOR;(b)搭载METEOR的双束电镜;(c)搭载自研发宽场荧光成像模块iFLM的赛默飞Aquilos 2双束电镜
Fig. 11. Commercial integrated cryo-FLM-FIB/SEM systems. (a) Widefield FLM module METEOR from Delmic for FIB/SEM; (b) FIB/SEM equipped with METEOR; (c) ThermoFisher Aquilos 2 FIB/SEM with self-developed widefield fluorescence imaging module iFLM
中国科学院生物物理研究所孙飞团队提出了一种新的宽场荧光成像与双束电镜集成方案ELI-TriScope[58](
![ELI-TriScope系统及应用[58]。(a)ELI-TriScope系统的三维结构示意图;(b)HeLa细胞中心粒原位结构](/richHtml/zgjg/2023/50/21/2107102/img_14.jpg)
图 12. ELI-TriScope系统及应用[58]。(a)ELI-TriScope系统的三维结构示意图;(b)HeLa细胞中心粒原位结构
Fig. 12. ELI-TriScope system and application[58]. (a) 3D schematic illustration of ELI-TriScope system; (b) in situ structure of centriole in HeLa cells
宽场荧光成像具有结构简单、成本低、成像速度快等优势,但由于缺少三维信息,无法对研究目标进行准确的三维定位。此外,细胞样品具有一定的厚度,在宽场成像中背景信号较高,会导致弱荧光信号淹没在背景中,从而无法识别。为了获得目标的三维定位信息和更高的光学成像质量,中国科学院生物物理研究所徐涛课题组、纪伟课题组和北京大学郭强课题组合作,研发出光电关联激光共聚焦双束电镜系统CLIEM[60](
![CLIEM系统及应用[60]。(a)CLIEM系统三维结构示意图;(b)线粒体(M)-脂滴(LD)互作位点的原位结构,箭头所指是在互作面上发现的丝状结构,标尺为50 nm;(c)HeLa细胞中心粒及周边结构的渲染图](/richHtml/zgjg/2023/50/21/2107102/img_15.jpg)
图 13. CLIEM系统及应用[60]。(a)CLIEM系统三维结构示意图;(b)线粒体(M)-脂滴(LD)互作位点的原位结构,箭头所指是在互作面上发现的丝状结构,标尺为50 nm;(c)HeLa细胞中心粒及周边结构的渲染图
Fig. 13. CLIEM system and application[60]. (a) 3D schematic illustration of CLIEM system; (b) in situ structure of contact site between mitochondria (M) and lipid droplet (LD) with tethering structures discovered on interaction surface shown by arrows and scalebar is 50 nm; (c) rendering of HeLa cell centriole and surrounding structure
3 总结和展望
随着近年来细胞原位结构解析领域的快速发展,cryo-ET已经成为人们在细胞原位探索生命奥秘的一项前沿技术。由于具有高保真的冷冻制样方法以及高分辨的三维解析能力,cryo-ET可以在近乎天然的生理状态下观察细胞内部的超微结构,在纳米尺度揭示细胞原位的生理过程,具有非常广阔的应用前景。然而,作为一项新兴技术,cryo-ET仍面临诸多技术挑战,样品制备便是其中之一。
冷冻生物样品减薄是cryo-ET的一个关键步骤,减薄的效率和成功率直接决定cryo-ET实验的成败。作为目前主流的冷冻生物样品减薄技术,FIB切割具有操作简便、精度高、切片质量好等特点,在透射电镜样品制备中发挥了至关重要的作用。但传统的FIB盲切方法无法对特定的细胞结构进行定点减薄,盲切的低效率和低成功率已经成为阻碍cryo-ET发展的瓶颈。
最新发展出的光电关联荧光导航FIB减薄技术赋予了FIB“视觉”的能力,通过荧光成像可以精确定位研究目标,再利用光电关联锁定目标在FIB图像中的位置,解决了FIB定点切割的难题。这项技术目前主要有两种实现方案:分体式方案和集成式方案。分体式方案比较灵活,可以通过在显微镜上加装商业化冷台实现冷冻光学显微镜成像,成本较低。但分体式方案的工作流程较为复杂,样品传递次数多,容易造成样品的冰污染、升温或损坏,还需使用配准标记物和配准软件才能实现光电联用,配准精度和工作效率都比较低。集成式方案是将冷冻光镜与冷冻双束电镜整合成一套设备,操作简便,工作效率高,样品转移次数少,具有替代分体式方案的潜力。但集成式方案成本较高,双束电镜升级光镜模块需要根据具体电镜型号进行定制,升级周期较长。另外,受限于电镜外挂模块的体积和质量,目前只有宽场和共聚焦两种成像模式可供选择。
降低成本、提供更加丰富的光学成像模式并实现工作流程自动化是未来集成式荧光导航FIB减薄的发展方向。在成本控制方面,相信通过普及此技术和增加光镜模块装机量,前期的研发成本将减小,光镜有望逐渐成为双束电镜的标配模块之一。在成像模式升级方面,可将共聚焦成像升级为Airyscan或受激辐射损耗(STED)[74]成像,将宽场成像升级为单分子定位成像(SMLM)[75],从而提高光学成像的分辨率。还可将共聚焦成像与宽场成像结合并拓展为多模态成像,可适应更加广泛的应用场景。此外,还可利用高空间带宽成像[76]或超分辨结构光照明成像[77]等成像模式,扩大成像的视野。在自动化方面,FIB自动减薄已经实现[78],未来可在此基础上将光学成像融合进来,通过人工智能等软件算法自动识别研究目标并完成光电配准,有望实现批量自动化的荧光导航FIB定点减薄。
总之,荧光导航FIB减薄技术将特异性荧光成像与高质量FIB切割结合起来,为定点制备透射电镜生物样品切片提供了新的解决方案,可用来精准研究细胞内部特定的超微结构,拓展了cryo-ET的应用范围,必将推动原位结构生物学的发展。
[1] Turk M, Baumeister W. The promise and the challenges of cryo-electron tomography[J]. FEBS Letters, 2020, 594(20): 3243-3261.
[2] Hylton R K, Swulius M T. Challenges and triumphs in cryo-electron tomography[J]. iScience, 2021, 24(9): 102959.
[3] De Rosier D J, Klug A. Reconstruction of three dimensional structures from electron micrographs[J]. Nature, 1968, 217(5124): 130-134.
[4] Dubochet J, Lepault J, Freeman R, et al. Electron microscopy of frozen water and aqueous solutions[J]. Journal of Microscopy, 1982, 128(3): 219-237.
[5] Adrian M, Dubochet J, Lepault J, et al. Cryo-electron microscopy of viruses[J]. Nature, 1984, 308(5954): 32-36.
[6] Dubochet J, Adrian M, Lepault J, et al. Emerging techniques: Cryo-electron microscopy of vitrified biological specimens[J]. Trends in Biochemical Sciences, 1985, 10(4): 143-146.
[7] Mahamid J, Baumeister W. Cryo-electron tomography: the realization of a vision[J]. Microscopy and Analysis, 2012, 26: 45-48.
[8] Beck M, Baumeister W. Cryo-electron tomography: can it reveal the molecular sociology of cells in atomic detail?[J]. Trends in Cell Biology, 2016, 26(11): 825-837.
[9] Oikonomou C M, Jensen G J. Cellular electron cryotomography: toward structural biology in situ[J]. Annual Review of Biochemistry, 2017, 86: 873-896.
[10] Lučič V, Rigort A, Baumeister W. Cryo-electron tomography: the challenge of doing structural biology in situ[J]. The Journal of Cell Biology, 2013, 202(3): 407-419.
[11] Shi S, Sun S, Andrews S B, et al. Thickness measurement of hydrated and dehydrated cryosections by EELS[J]. Microscopy Research and Technique, 1996, 33(3): 241-250.
[12] Marko M, Hsieh C, Schalek R, et al. Focused-ion-beam thinning of frozen-hydrated biological specimens for cryo-electron microscopy[J]. Nature Methods, 2007, 4(3): 215-217.
[13] Hsieh C, Schmelzer T, Kishchenko G, et al. Practical workflow for cryo focused-ion-beam milling of tissues and cells for cryo-TEM tomography[J]. Journal of Structural Biology, 2014, 185(1): 32-41.
[14] Al-Amoudi A, Studer D, Dubochet J. Cutting artefacts and cutting process in vitreous sections for cryo-electron microscopy[J]. Journal of Structural Biology, 2005, 150(1): 109-121.
[15] Han H M, Zuber B, Dubochet J. Compression and crevasses in vitreous sections under different cutting conditions[J]. Journal of Microscopy, 2008, 230(2): 167-171.
[16] Villa E, Schaffer M, Plitzko J M, et al. Opening windows into the cell: focused-ion-beam milling for cryo-electron tomography[J]. Current Opinion in Structural Biology, 2013, 23(5): 771-777.
[17] Rigort A, Plitzko J M. Cryo-focused-ion-beam applications in structural biology[J]. Archives of Biochemistry and Biophysics, 2015, 581: 122-130.
[18] Kuba J, Mitchels J, Hovorka M, et al. Advanced cryo-tomography workflow developments-correlative microscopy, milling automation and cryo-lift-out[J]. Journal of Microscopy, 2021, 281(2): 112-124.
[19] Sartori A, Gatz R, Beck F, et al. Correlative microscopy: bridging the gap between fluorescence light microscopy and cryo-electron tomography[J]. Journal of Structural Biology, 2007, 160(2): 135-145.
[20] Briegel A, Chen S Y, Koster A J, et al. Correlated light and electron cryo-microscopy[J]. Methods in Enzymology, 2010, 481: 317-341.
[21] de Boer P, Hoogenboom J P, Giepmans B N G. Correlated light and electron microscopy: ultrastructure lights up![J]. Nature Methods, 2015, 12(6): 503-513.
[22] Karreman M A, Hyenne V, Schwab Y, et al. Intravital correlative microscopy: imaging life at the nanoscale[J]. Trends in Cell Biology, 2016, 26(11): 848-863.
[23] van Driel L F, Valentijn J A, Valentijn K M, et al. Tools for correlative cryo-fluorescence microscopy and cryo-electron tomography applied to whole mitochondria in human endothelial cells[J]. European Journal of Cell Biology, 2009, 88(11): 669-684.
[24] Jun S M, Ke D X, Debiec K, et al. Direct visualization of HIV-1 with correlative live-cell microscopy and cryo-electron tomography[J]. Structure, 2011, 19(11): 1573-1581.
[25] Liu B, Xue Y H, Zhao W, et al. Three-dimensional super-resolution protein localization correlated with vitrified cellular context[J]. Scientific Reports, 2015, 5(1): 1-11.
[26] Li S G, Ji G, Shi Y, et al. High-vacuum optical platform for cryo-CLEM (HOPE): a new solution for non-integrated multiscale correlative light and electron microscopy[J]. Journal of Structural Biology, 2018, 201(1): 63-75.
[27] Moser F, Pražák V, Mordhorst V, et al. Cryo-SOFI enabling low-dose super-resolution correlative light and electron cryo-microscopy[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(11): 4804-4809.
[28] Rigort A, Kirmse R, Doring V, et al. Imaging of vitrified biological specimens by confocal cryo-fluorescence microscopy and cryo-FIB/SEM tomography[J]. Microscopy and Microanalysis, 2015, 21(S3): 1121-1122.
[29] Hoffman David P, Gleb S, Shan X C, et al. Correlative three-dimensional super-resolution and block-face electron microscopy of whole vitreously frozen cells[J]. Science, 2020, 367(6475): eaaz5357.
[30] Rigort A, Villa E, Bäuerlein F J B, et al. Integrative approaches for cellular cryo-electron tomography: correlative imaging and focused ion beam micromachining[J]. Methods in Cell Biology, 2012, 111: 259-281.
[31] HuangB Q, YeungE C. Chemical and physical fixation of cells and tissues: an overview[M]∥Yeung E C T, Stasolla C, Sumner M J, et al. Plant microtechniques and protocols. Cham: Springer, 2015: 23-43.
[32] MoorH. Theory and practice of high pressure freezing[M]∥Steinbrecht R A, Zierold K. Cryotechniques in biological electron microscopy. Heidelberg: Springer, 1987: 175-191.
[33] GalwayM E, HeckmanJ W, Jr, HydeG J, et al. Advances in high-pressure and plunge-freeze fixation[M]∥Methods in cell biology. Amsterdam: Elsevier, 1995: 3-19.
[34] Mielanczyk L, Matysiak N, Michalski M, et al. Closer to the native state. Critical evaluation of cryo-techniques for Transmission Electron Microscopy: preparation of biological samples[J]. Folia Histochemica et Cytobiologica, 2014, 52(1): 1-17.
[35] Kanno H, Speedy R J, Angell C A. Supercooling of water to -92 ℃ under pressure[J]. Science, 1975, 189(4206): 880-881.
[36] Handley D A, Alexander J T, Chien S. The design and use of a simple device for rapid quench-freezing of biological samples[J]. Journal of Microscopy, 1981, 121(3): 273-282.
[37] Dubochet J, McDowall A W, Menge B, et al. Electron microscopy of frozen-hydrated bacteria[J]. Journal of Bacteriology, 1983, 155(1): 381-390.
[38] McDowall A W, Chang J J, Freeman R, et al. Electron microscopy of frozen hydrated sections of vitreous ice and vitrified biological samples[J]. Journal of Microscopy, 1983, 131(1): 1-9.
[39] McDowall A W, Hofmann W, Lepault J, et al. Cryo-electron microscopy of vitrified insect flight muscle[J]. Journal of Molecular Biology, 1984, 178(1): 105-111.
[40] Iancu C V, Tivol W F, Schooler J B, et al. Electron cryotomography sample preparation using the Vitrobot[J]. Nature Protocols, 2006, 1(6): 2813-2819.
[41] Dobro M J, Melanson L A, Jensen G J, et al. Plunge freezing for electron cryomicroscopy[J]. Methods in Enzymology, 2010, 481: 63-82.
[42] Swanson L W. Liquid metal ion sources: mechanism and applications[J]. Nuclear Instruments and Methods in Physics Research, 1983, 218(1/2/3): 347-353.
[43] Smith N S, Skoczylas W P, Kellogg S M, et al. High brightness inductively coupled plasma source for high current focused ion beam applications[J]. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures, 2006, 24(6): 2902-2906.
[44] Orloff J. High‐resolution focused ion beams[J]. Review of Scientific Instruments, 1993, 64(5): 1105-1130.
[45] GiannuzziL A, StevieF A. Introduction to focused ion beams instrumentation, theory, techniques and practice[M]. Boston: Springer, 2004.
[47] Dubochet J, Booy F P, Freeman R, et al. Low temperature electron microscopy[J]. Annual Review of Biophysics and Bioengineering, 1981, 10: 133-149.
[48] Marko M, Hsieh C, Moberlychan W, et al. Focused ion beam milling of vitreous water: prospects for an alternative to cryo-ultramicrotomy of frozen-hydrated biological samples[J]. Journal of Microscopy, 2006, 222(1): 42-47.
[49] Rigort A, Bäuerlein F J B, Villa E, et al. Focused ion beam micromachining of eukaryotic cells for cryoelectron tomography[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(12): 4449-4454.
[50] Rigort A, Bäuerlein F J B, Leis A, et al. Micromachining tools and correlative approaches for cellular cryo-electron tomography[J]. Journal of Structural Biology, 2010, 172(2): 169-179.
[51] Schaffer M, Engel B D, Laugks T, et al. Cryo-focused ion beam sample preparation for imaging vitreous cells by cryo-electron tomography[J]. Bio-protocol, 2015, 5(17): e1575.
[52] Medeiros J M, Böck D, Weiss G L, et al. Robust workflow and instrumentation for cryo-focused ion beam milling of samples for electron cryotomography[J]. Ultramicroscopy, 2018, 190: 1-11.
[53] Hayles M F, Stokes D J, Phifer D, et al. A technique for improved focused ion beam milling of cryo-prepared life science specimens[J]. Journal of Microscopy, 2007, 226(3): 263-269.
[54] Wolff G, Limpens R W A L, Zheng S, et al. Mind the gap: micro-expansion joints drastically decrease the bending of FIB-milled cryo-lamellae[J]. Journal of Structural Biology, 2019, 208(3): 107389.
[55] Kaufmann R, Hagen C, Grünewald K. Fluorescence cryo-microscopy: current challenges and prospects[J]. Current Opinion in Chemical Biology, 2014, 20: 86-91.
[56] Gorelick S, Buckley G, Gervinskas G, et al. PIE-scope, integrated cryo-correlative light and FIB/SEM microscopy[J]. eLife, 2019, 8: e45919.
[57] Smeets M, Bieber A, Capitanio C, et al. Integrated cryo-correlative microscopy for targeted structural investigation in situ[J]. Microscopy Today, 2021, 29(6): 20-25.
[58] Li S G, Wang Z Y, Jia X, et al. ELI trifocal microscope: a precise system to prepare target cryo-lamellae for in situ cryo-ET study[J]. Nature Methods, 2023, 20(2): 276-283.
[59] Arnold J, Mahamid J, Lucic V, et al. Site-specific cryo-focused ion beam sample preparation guided by 3D correlative microscopy[J]. Biophysical Journal, 2016, 110(4): 860-869.
[60] Li W X, Lu J, Xiao K, et al. Integrated multimodality microscope for accurate and efficient target-guided cryo-lamellae preparation[J]. Nature Methods, 2023, 20(2): 268-275.
[61] Sexton D L, Burgold S, Schertel A, et al. Super-resolution confocal cryo-CLEM with cryo-FIB milling for in situ imaging of Deinococcus radiodurans[J]. Current Research in Structural Biology, 2022, 4: 1-9.
[62] Wu G H, Mitchell P G, Galaz-Montoya J G, et al. Multi-scale 3D cryo-correlative microscopy for vitrified cells[J]. Structure, 2020, 28(11): 1231-1237.
[63] Schwartz C L, Sarbash V I, Ataullakhanov F I, et al. Cryo-fluorescence microscopy facilitates correlations between light and cryo-electron microscopy and reduces the rate of photobleaching[J]. Journal of Microscopy, 2007, 227(2): 98-109.
[64] Le Gros M A, McDermott G, Uchida M, et al. High-aperture cryogenic light microscopy[J]. Journal of Microscopy, 2009, 235(1): 1-8.
[65] Xu X J, Xue Y H, Tian B Y, et al. Ultra-stable super-resolution fluorescence cryo-microscopy for correlative light and electron cryo-microscopy[J]. Science China Life Sciences, 2018, 61(11): 1312-1319.
[66] Li W X, Stein S C, Gregor I, et al. Ultra-stable and versatile widefield cryo-fluorescence microscope for single-molecule localization with sub-nanometer accuracy[J]. Optics Express, 2015, 23(3): 3770-3783.
[67] Schorb M, Gaechter L, Avinoam O, et al. New hardware and workflows for semi-automated correlative cryo-fluorescence and cryo-electron microscopy/tomography[J]. Journal of Structural Biology, 2017, 197(2): 83-93.
[68] Huff J, Bergter A, Birkenbeil J, et al. The new 2D Superresolution mode for ZEISS Airyscan[J]. Nature Methods, 2017, 14(12): 1223.
[71] Kukulski W, Schorb M, Welsch S, et al. Correlated fluorescence and 3D electron microscopy with high sensitivity and spatial precision[J]. Journal of Cell Biology, 2011, 192(1): 111-119.
[72] Kukulski W, Schorb M, Welsch S, et al. Precise, correlated fluorescence microscopy and electron tomography of lowicryl sections using fluorescent fiducial markers[J]. Methods in Cell Biology, 2012, 111: 235-257.
[73] Fukuda Y, Schrod N, Schaffer M, et al. Coordinate transformation based cryo-correlative methods for electron tomography and focused ion beam milling[J]. Ultramicroscopy, 2014, 143: 15-23.
[74] Hell S W, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy[J]. Optics Letters, 1994, 19(11): 780-782.
[75] Lelek M, Gyparaki M T, Beliu G, et al. Single molecule localization microscopy[J]. Nature Reviews Methods Primers, 2021, 1(1): 39.
[78] Zachs T, Schertel A, Medeiros J, et al. Fully automated, sequential focused ion beam milling for cryo-electron tomography[J]. eLife, 2020, 9: e52286.
Article Outline
李尉兴, 卢婧, 肖珂, 纪伟. 荧光导航冷冻聚焦离子束减薄技术的研究进展[J]. 中国激光, 2023, 50(21): 2107102. Weixing Li, Jing Lu, Ke Xiao, Wei Ji. Recent Developments in Fluorescence-Guided Cryogenic Focused-Ion-Beam Milling[J]. Chinese Journal of Lasers, 2023, 50(21): 2107102.






