植入式多模态神经接口前沿进展  下载: 1325次封面文章【增强内容出版】
下载: 1325次封面文章【增强内容出版】
The nervous system is composed of different types of neurons connected in a network. Neurons communicate with each other through electrochemical signals, and this dynamic interaction of neurons is the internal driving force of human perception, cognition, and behavior. Deciphering and understanding the meaning of various complex neural activities is of great significance in the frontiers of neurological disease diagnosis and treatment, neurological rehabilitation, fundamental brain science, and several brain-computer interface applications. To achieve this, it is critical to develop advanced neural interfaces capable of interacting with the dynamic neural activities and nervous system. Fundamental research in this field has rapidly increased over the past few decades as a result of advancements in neuroscience and neurotechnology. This research includes the development of innovative neural recording and modulation tools that have provided researchers with an early glimpse into previously unanswerable questions, such as determining how the mind works, or which have been recognized and funded by a host of initiatives. In recent years, the United States, European countries, Japan, and China have launched their own brain initiatives to support this emerging field. In the future, in order to completely understand the complex structural and functional nervous system, more powerful tools must be developed to record, transmit, and modulate signals using multiple approaches. These tools must have the ability to manipulate neuron cell types specifically while minimizing side effects such as “the observer effect.”
Neurological disorders affect more than one billion people worldwide, accounting for 7% of the total global disease burden, and this number is expected to increase substantially as human life expectancy increases and with increased population aging. This is largely due to neuropsychiatric diseases (including Alzheimer's disease, Parkinson's disease, epilepsy, and so on) and cerebrovascular diseases, which impose a heavy burden on society and individuals, while also promoting advances and developments in neuroengineering, biomedical science, and technology. Currently, the treatment of these diseases relies primarily on drug therapy or implantable electrical stimulation devices, such as injecting current into the target tissue through metal electrodes to activate or suppress the action potential of neurons, as well as to achieve therapeutic purposes, including using cochlear implants, deep brain stimulators, spinal cord electrical stimulation, and visual prostheses to reduce symptoms or restore nerve functions.
With the enrichment of multiple neural modalities, neural technologies and tools have been increasingly augmented, and have been widely used to collect neuron activities in vivo from individual neurons to neuron populations in different brain areas, with a variety of signal recording and modulation manners (Fig. 1). In addition, advances in genetic engineering, especially optogenetics, allow us to continuously control specific types of neurons with high accuracy and fidelity over a short period. The rapid development of genetically encoded neural probes provides new avenues for real-time and high-speed neural recording. Various optical, electrical, and chemical tools have been developed to record and modulate neural activities. Currently, the monolithic integration of multiple functional features has become a pressing demand and challenge in neural engineering, while flexible neural implants are expected to establish seamless integration with the soft biological tissue and achieve a high-bandwidth close-loop interaction with the nervous system. It will provide a powerful tool for identifying complex neural circuits, as well as diagnosing and treating neurological diseases. In order to more accurately understand the brain neural network and its working mechanism, and to treat neurological diseases with high selectivity, it is necessary to simultaneously monitor neural activities with high spatial and temporal resolution. The combination of electrophysiological and optical methods (for example, two-photon imaging and electrophysiological recording) can maximize the synergistic effect of the two methods, making up for the shortcomings of each method.
Implantable multimodal neural interfaces integrate these approaches by maximizing their benefits and efficacy, providing neuroscientists with new access to the brain and revolutionizing applications such as the treatment and rehabilitation of neurological diseases. In order to achieve this, we need to understand the various neurotechnologies individually, how they function, as well as how they work together. Implantable neural interfaces have already been successfully employed in the long-term stable interrogation of large-scale neural activities and clinically restoring sensorimotor function in disabilities. However, they are limited in the long run by poor biocompatibility, mechanical mismatches between the device and neural tissue, and the risk of chronic inflammatory reactions after implantation. In addition, traditional neural probes are limited by spatial and temporal resolution and scalabilities, and still face challenges in the study of large-scale neural networks in situ (Fig. 1). To this end, long-term stability is achieved by matching the mechanical properties of the implanted device with those of the internal biological tissue. High spatial and temporal resolution and even specificity can be obtained by reducing the feature size of implants and mimicking the structural morphology of neurons. Multimodal neural interfaces are currently emerging, in particular, a variety of clinical multimodal implantable devices have been developed to treat neurodegenerative diseases such as Parkinson's disease, epilepsy, and depression. However, traditional device designs, such as electrophysiological readout, fluorescence cell imaging, and the structural dynamics of the brain, may conflict with each other during different data acquisition processes. Severe electrophysiological signal contamination caused by photoelectric (magnetic) artifacts can also occur. Over the past decade, there have been efforts to address these challenges, and many excellent results have emerged regarding the latest advances in neural technologies and applications.
With the development of neural probe structures and materials, as well as the innovation of synthetic technologies for nanoparticles, dye molecules, and genetically encoded proteins, it is expected that neural technology will continue to be developed toward the limits of the lifetime, localization and specificity of neural recording and stimulation, and will eventually blur the boundary between living biological tissue and physical equipment and tools. These cutting-edge neural technologies, which combine advanced optical and nanoelectronic technologies, optogenetics, genetically encoded indicators, and acoustic and magnetic tools, provide us with unprecedented opportunities for new multimodal neural information interactions, with which powerful paradigms for multimodal inquiry of brain activity will be foreseen, and will even fundamentally alter how brain activity maps to the physical world.
1 引言
神经系统由不同种类的神经元以网络结构形式连接组成,它们之间通过电化学信号相互交流,这种动态相互作用是人类感知、认知和行为的内在基础;破译和理解各种神经活动模式,对于神经疾病诊疗、神经康复、基础脑科学和脑机交互等前沿领域具有重大意义,因此开发和探索能与动态神经系统交互的神经接口技术至关重要。在过去的几十年中,神经科学技术不断进步,新型神经记录和调控工具的开发促进了神经科学特别是脑科学研究爆炸式的增长。美国、欧洲各国、日本和中国等相继推出具有各自特色的脑计划 [1-2]。为了充分理解大脑神经系统复杂的结构和功能,必须开发新的工具以记录、传输和调节多种模态的信号,这些神经工具必须具备对不同类型的神经元进行特异性操作的能力,并应尽量减少诸如“观察者效应”之类的副作用[3]。
神经疾病正影响着全球10亿多人,占全球疾病总负担的7%,随着人类预期寿命的增加和人口老龄化进程的加剧,这一数字会大幅增加。这主要归因于神经精神疾病(包括阿尔茨海默病、帕金森病和癫痫等)和脑血管疾病,它们给社会和经济造成沉重负担,同时也促使神经工程及医学科学和技术发展[3-5]。目前这些疾病的治疗主要依赖于药物治疗或植入式电刺激装置,如通过金属电极向目标组织注入电流,诱导或抑制神经元动作电位,达到治疗目的,包括通过人工耳蜗、脑深部电刺激器、脊髓电刺激和视觉假体等减轻患者症状或恢复功能[6-10]。
神经元是神经系统中最基本的结构和功能单位,神经系统的信息传递是通过神经信号来实现的,即神经的兴奋传递。本质上,神经信号包括神经元内的电信号(动作电位)和神经元之间的化学信号(神经递质),本文主要探讨神经电信号采集、传输和调制等。早期的神经元胞外电记录工具主要是金属微丝和玻璃微电极等[11],现代材料科学和先进制造技术的发展为生物体与外界物理设备的交互开辟了全新途径[12-16]。特别是在过去的十年中,生物电子设备在神经元活动探测和分析中的灵敏度、精度和空间分辨率取得了一系列重大进展,科研人员已经实现了在特定区域以不同分辨率在多个尺度和模式上对生命活动进行读取、分析和控制[17-19]。生物电子设备与神经系统的双向交互使科研人员能够监测大脑网络和神经元的状态,并通过调节它们来治疗疾病或修复感知觉和运动功能。为了提供稳定有效的神经接口,这些工具必须将柔软、活性和高动态的神经组织与刚性、静态的微电子和医疗设备连接起来。为了弥合固有的生物与非生物的界面失配,我们需要了解这些物理工具与活体生物组织的相互作用,并利用神经细胞的内在机制探索将信息传入和传出大脑的新方法[20]。
随着神经信号模式研究方法的发展,神经接口技术与工具也日益发展,并被广泛用于收集从在体单神经元到不同区域神经元群体的活动,已具备多种信号采集和调节模式,主要包括光、电、磁、声等种类,其信号采集的时间和空间分辨率也各不相同(
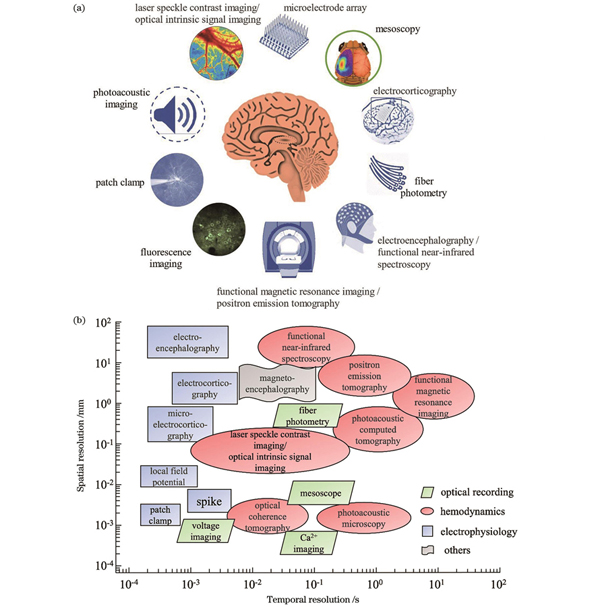
图 1. 不同模态神经接口示意图与时空分辨率。(a)不同种类神经接口示意图;(b)不同神经接口技术的时间和空间分辨率
Fig. 1. Schematics of neural interfaces with different modes and their spatial/temporal resolutions. (a) Schematic of different kinds of neural interfaces; (b) spatial and temporal resolutions of different neural interface technologies
![用于神经记录的光学和电生理方法原理图。(a)结合了双光子荧光扫描显微成像和同步电生理记录的多模态神经记录系统示意图;(b)荧光图像分析以及提取出来的钙信号[37];(c)基于神经探针的小鼠胞外电生理记录原理示意图[35]](/richHtml/zgjg/2023/50/15/1507301/img_05.jpg)
图 2. 用于神经记录的光学和电生理方法原理图。(a)结合了双光子荧光扫描显微成像和同步电生理记录的多模态神经记录系统示意图;(b)荧光图像分析以及提取出来的钙信号[37];(c)基于神经探针的小鼠胞外电生理记录原理示意图[35]
Fig. 2. Principles of optical and electrical methods for neural recording. (a) Schematic of multimodal neural recording system combining two-photon fluorescence scanning microscopy imaging and synchronous electrophysiological recording; (b) fluorescence image analysis and extracted calcium signals[37]; (c) schematics of extracellular electrophysiological recording in mice based on neural probe[35]
植入式多模态神经接口集成了上述多种方法和工具,能最大限度地发挥各种方法的优势和功效,为神经科学家提供了全新的访问大脑的途径,并为神经疾病治疗和康复等应用带来变革,目前已在长期稳定操控大规模神经活动以及恢复残疾人的感知觉和运动功能等方面取得了一定成功,但它们仍然受到生物相容性差、设备与神经组织之间的机械失配以及植入后出现慢性炎症反应风险的影响。此外,传统神经探针受限于时空分辨率和规模,在研究原位大规模神经网络时,依然面临诸多挑战。近年来也涌现出了诸多解决方案,例如通过将植入器件与内源生物组织的机械特性匹配,实现了长期稳定的生物界面;通过减小植入物的特征尺寸并模仿神经元的结构形貌,可以获取高时空分辨率甚至是特异选择性的神经记录[20]。多模态神经接口技术方兴未艾,临床上多模态植入式设备已开始应用于神经退行性疾病治疗,但也存在诸多问题,例如电生理信号读出、荧光细胞成像或大脑的结构动力学等不同数据采集过程中可能出现电磁伪影、信号串扰等冲突[38]。本文旨在讨论光学与电子学方法在神经科学和疾病诊断治疗中的应用,并介绍了综合这两种方法的最新研究,重点探讨了光电多模态植入设备和系统存在的问题和解决方案及其在未来生物医学研究应用中的重要性。
2 多种模态神经记录与调控的原理
多种模态神经接口主要包括运用光学、电学、磁学、声学以及化学药物输送等方法来记录和调控神经活动[22],其中光学神经接口主要包括神经活动的光学记录和光学刺激,根据是否应用外源材料(包括光敏性的基因、纳米材料、染料化合物等)分为外源和内源方法[39]:外源光学记录的主要原理是通过分子合成技术或基因工程将荧光信号与神经元局部电位关联起来,并通过光学检测手段来观测和记录来自指定“活性”波长的荧光信号。荧光生物探针种类繁多且在神经科学中应用广泛,一般包括合成型、基因编码型和混合型(使用合成染料和基因编码蛋白的组合)等[40-44],目前结合大规模单光子或多光子成像,已能读取清醒的、与动物行为学关联的神经环路活动[45-47]。外源光学刺激则包括基于光化学作用的光活性分子、基于光热和光伏作用的纳米材料以及需要基因修饰的光遗传刺激;内源光学信号(IOS)记录包括直接测量光与神经组织相互作用的散射特性[48]和间接测量与大脑活动相关的标记物质的浓度变化两类[49],而内源光学刺激主要利用某些神经元本身对特定光条件下的敏感特性进行光学调控,目前包括飞秒激光刺激和红外神经刺激(INS)等。基于电学的神经接口技术一直是基础神经科学和转化医学最重要的神经技术之一,在脑科学基础研究、生物电子医疗和脑机融合等前沿热点科学领域起着至关重要的作用。常用的神经电生理信号包括脑电图(EEG)、脑皮层电图(ECoG)、局部场电位(LFP)、动作电位(AP)或“峰电位”信号等,它们主要通过神经电极进行采集,而植入式神经电极及相关技术因具有亚毫秒量级的时间分辨率和在体检测单个神经元的电信号的能力而在现代神经接口应用中备受青睐。本文主要探讨当前光学和电学神经接口的前沿进展,包括基于基因工程技术的外源性光学记录和刺激、血流动力学成像及植入式神经电极记录和刺激等方法原理。
2.1 荧光分子探针与光遗传学工具简介
基因编码荧光指示剂能对各种神经活动事件作出响应,被广泛用于检测囊泡释放、神经递质/调质浓度[50]、跨膜电压、细胞内钙动力学及信号和代谢活动的变化等[51-53],这些信息是电极记录和功能磁共振成像不能获取的。基因编码的指示剂还可以对特定神经元亚群信息进行采样,研究学习记忆、活动事件、大脑发育或疾病等过程中神经活动随时间的变化并稳定表达。本文主要对神经电活动相关的钙离子指示剂(GECI)和跨膜电压指示剂(GEVI)作简单介绍。
钙离子在细胞兴奋性和细胞内信号通路中扮演重要角色,钙离子信号与神经元胞体、树突膜电位的相关性已通过细胞内同步电生理记录得到证实,对钙离子信号的观测和记录在神经科学研究中至关重要[54]。使用Ca2+指示剂对神经活动进行成像最初是由Tsien课题组实现的[55-56],目前已被广泛用于健康大脑[57]和脑疾病研究[58]。相对于合成指示染料,GECI更具优势,可用来进行包括特定细胞类型的遗传靶向、病毒介导的传递以及创建稳定表达GECI的转基因生物等。Tsien课题组于1997年报道了基于Förster共振能量转移(FRET)的GECI[59]。该团队在1999年研发出具有更强钙信号响应的基于单色荧光基团的GECI[60],随后经过多个团队不断改进,第一个被广泛用于确定细胞群中神经元活动成像的GECI是GCaMP3[61],现今已发展为第8代GCaMP变体[62-63],而目前最常用的仍是GCaMP6。GECI通常具有钙结合结构域和钙浓度敏感构象[59],例如GCaMP由绿色荧光蛋白(GFP)、钙调蛋白(CaM)和肌球蛋白轻链激酶的一段肽段序列M13构成。当有Ca2+结合CaM时,CaM发生构象变化,其轻链区可以结合M13,在特定波长的光照下GFP发色团的质子化作用增强、吸光度增加,从而使GFP发出强烈的荧光,如
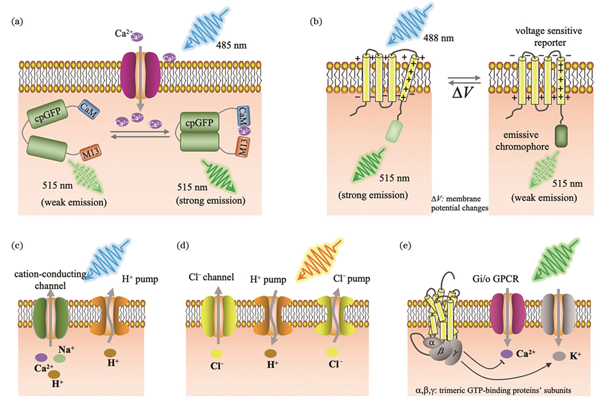
图 3. 基因编码的光学神经记录方法和光遗传调控方法示意图。(a)基因编码的Ca2+信号成像;(b)第一代基因编码的电压指示剂原理示意图;(c)基于视紫红质的兴奋性泵和通道;(d)由氯离子传导通道视紫红质、质子和氯离子泵组成的抑制性视蛋白;(e)偶联到抑制性Gi/o信号通路的G蛋白偶联受体
Fig. 3. Schematics of optical neural recording methods and optogenetic manipulation methods based on genetically encoding. (a) Genetically encoded Ca2+ imaging; (b) principle of first genetically encoded voltage indicator; (c) excitatory pump and channel based on rhodopsin; (d) inhibitory opsin composed of chloride-conducting channel rhodopsin, protons, and chloride pumps; (e) G-protein-coupled receptors coupled to inhibitory Gi/o pathway
钙离子信号衰减较慢,并不能充分表征毫秒量级神经元的动作电位,且无法表征阈下膜电位,而细胞膜电位的变化是神经活动的另一重要指标,因此研究人员还提出了利用细胞膜电位改变作为信号进行荧光成像的方法(即电压成像技术)以解决上述问题。电压成像技术的原理是将电压敏感域和荧光蛋白连接起来,当膜电位改变时,电压敏感蛋白结构改变,从而发光特性改变。GEVI则是通过基因修饰靶向特定细胞和蛋白表达,将荧光蛋白与其他蛋白质耦合(如嵌入细胞膜中的电压敏感蛋白),使得这些耦合的荧光蛋白能够响应于膜电位,从而改变构象并发光[71-72],如
GECI和GEVI等基因编码的荧光指示剂已经彻底改变了系统神经科学,使得研究神经元亚群和整个大脑神经活动随时间的变化,以及对突触激活的细胞亚型反应的神经编码成为可能。目前囊泡融合、神经递/调质等基因编码指示剂正在快速发展,未来基因编码的细胞活动指示剂将变得更具多样性,例如将神经活动可视化和光遗传操控结合,有望实现对同一细胞形态学、连通性和大分子分布的解剖观测,从而理解大脑特定环路功能的最核心部分[50,52,86]。
Crick研究组提出神经科学面临的主要挑战之一是控制大脑中的一种细胞同时保持其他细胞不变,并预测光可能是合适的控制神经元的工具[87]。2002年,Nagel等[88-89]提出视紫质通道蛋白ChR1和ChR2。2005年,Boyden等[90]首次将ChR2转入到大鼠神经元中,并使用蓝光激发实现了毫秒量级的神经元激活,这种基于光敏感蛋白的神经调控技术被称为光遗传学。如
经过近二十年的发展,光遗传学技术已经为神经科学带来前所未有的改变,其丰富的种类,包括基于光门控离子泵、阳离子和阴离子传导通道、GPCR蛋白和光激活酶的工具等为订制化的神经科学研究提供了极大的灵活性。然而由于自然状态下的视蛋白存在光诱发效率低、信号弱、无法实时开关等缺点,近年来该领域的研究热点是视蛋白设计与合成,即对视蛋白的离子选择性、光谱特性、动力学特征、光敏性和离子电导等进行改进[97-102]。此外,与基因编码的荧光探针类似,光遗传学研究也需要将激活光谱红移[75,103],因为长波长有助于减少光散射和光损伤,并提高穿透深度,并且当视蛋白与其他工具(如GECI和GEVI)结合使用时,有助于消除Ca2+信号串扰。因此在特定光遗传学实验时,需要综合考虑视蛋白的离子选择性、动力学、作用光谱和光电流幅度等因素。
2.2 血流动力学成像原理简介
虽然基因编码的荧光指示剂可以对神经活动进行成像,但难以在临床上应用。相比之下,血流变化与神经活动息息相关,例如星形胶质细胞和小胶质细胞等脑细胞与神经元和血管相互作用以维持健康的大脑活动和代谢,因此神经血管耦合提供了一种通过测量血流来反映神经活动的方法。在神经元活动期间,神经元和神经胶质细胞释放的血管活性分子和离子控制局部微脉管系统的直径,从而产生血流变化。同时,活跃的脑细胞消耗能量,导致从血管到脑组织的氧摄取量的增加[104-105]。因此对血流量和氧合的测量可以间接反映神经元的活动。此外,这些测量本身也很重要,因为血流灌注和氧气在血管和组织(间质间隙)中的分压是健康和疾病的基础生理参数。对脑血流量、血容量和氧合敏感度的测量通常被称为“血流动力学”。已有较成熟的血流成像技术在临床中应用,如核磁共振成像、超声血流成像、激光散斑血流成像[106-107],这些方法通常不需要基因修饰和治疗,因此不存在安全性和伦理问题方面的挑战。
当前临床上最常用的血流动力学成像方式之一是功能性磁共振成像(fMRI),它是一种宏观非侵入性技术,利用具有顺磁特性的脱氧血红蛋白来产生信号,间接反应神经活动。人体大脑在响应某种指令时,神经元的活动会变得强烈,使得血流量随之发生变化。活跃的神经元消耗更多的氧气,导致这些大脑区域的氧气浓度降低,这种现象被称为血氧水平依赖性(BOLD)效应,一般可通过fMRI测量这种现象来反映神经活动。在这种情况下,fMRI依赖于血红蛋白氧载体磁性信息这一内源性信号。通常脱氧血红蛋白比含氧血红蛋白更具顺磁性,因而血氧变化可通过功能性磁共振成像检测到的磁对比度信号来检测。fMRI的空间分辨率可达到1 mm,然而,BOLD效应的发生需要时间,通常为5~8 s[108],这导致fMRI的时间分辨率不高,一般为1~2 s。
脑部血流量(CBF)还可通过激光散斑衬比血流成像(LSCI)[106]、可测量红细胞速度的双光子扫描成像[109-110]、光学相干层析成像(OCT)[111-112]、光声成像等方法[113-114]进行测量[
![主要血流动力学的光学成像方法。(a)人体脑皮层手术显微图像(左)和相应的散斑对比图像(右)[106];(b)双光子扫描成像对小鼠脑皮层血管的三维重建[109];(c)通过颅窗用基于相位的OCT血管造影术成像的小鼠大脑血管深度投影[113];(d)小鼠全脑皮层氧饱和度的声光显微成像[114];光学内源成像对血流动力学信号的(e)多通道混合和(f)分离成像[115];(g)人脑非侵入性近红外光谱功能成像示意图[116]](/richHtml/zgjg/2023/50/15/1507301/img_07.jpg)
图 4. 主要血流动力学的光学成像方法。(a)人体脑皮层手术显微图像(左)和相应的散斑对比图像(右)[106];(b)双光子扫描成像对小鼠脑皮层血管的三维重建[109];(c)通过颅窗用基于相位的OCT血管造影术成像的小鼠大脑血管深度投影[113];(d)小鼠全脑皮层氧饱和度的声光显微成像[114];光学内源成像对血流动力学信号的(e)多通道混合和(f)分离成像[115];(g)人脑非侵入性近红外光谱功能成像示意图[116]
Fig. 4. Major optical imaging methods for hemodynamics. (a) Photograph of human cortex captured by surgical microscope (left) and corresponding speckle contrast image (right)[106]; (b) three-dimensional reconstruction of cerebral vessels in rats by two-photon scanning imaging[109]; (c) projection of mouse cerebral vascular depth imaged through cranial windows using phase-based OCT angiography[113]; (d) acousto-optic microimaging of oxygen saturation in whole cerebral cortex of mice[114]; (e) multi-channel mixing and (f) separation imaging of hemodynamic signals by optical endogenous imaging[115]; (g) schematic of non-invasive NIR spectroscopic functional imaging of human brain[116]
除了使用血流速度测量之外,还可以利用含氧血红蛋白和脱氧血红蛋白吸收光谱的差异来检测神经活动,包括光学内源信号成像(OISI)[115][
光声成像技术,如光声断层成像(PACT)和显微成像(PAM)等,为血液动力学成像提供了新的解决方案。这是一种混合技术,生物组织吸收光子能量后温度升高,热膨胀导致压力波的产生,即产生声音信号,并利用超声波换能器检测这些信号[114-120]。其中,PACT能以数百微米的分辨率对小动物全身或人体器官进行深层成像;采用聚焦方式的PAM甚至可以达到光学分辨率,实现从亚细胞、细胞到浅层生物组织水平的成像,如血流灌注、氧合成像等。与电生理方法相比,这些可间接反映神经活动的光学测量方法具有更好的空间分辨率,可以以非接触的方式观察感兴趣区域,而且不会对组织和结构造成损伤破坏。
2.3 神经记录与调控光学系统简介
早在19世纪80年代,现代神经科学之父Cajal就利用光学显微镜观察基于Golgi染色法的脑组织切片,从而建立了“神经元学说”[11]。时至今日,使用光学方法和工具来记录和调控神经元活动已经成为神经科学研究的重要方式之一。21世纪以来,受益于新型荧光探针、成像器件和计算方法的快速发展,新的神经记录与调控方法也不断涌现,并已在视场、深度、时空分辨率等方面不断突破,已有许多国内外优秀的综述论文对此进行了归纳总结[38,121-126]。如
![不同面向活体小鼠的光学成像(或记录)与光遗传神经调控的方法示意图与实物照片。(a)标准的台式化显微成像与光遗传调控方法;(b)植入GRIN透镜用于深层脑区成像或植入微棱镜用于内测脑区成像;用于自由运动动物活体成像的(c)小型化头戴式微型显微镜系统及(d)光纤视镜和光纤光度记录系统;(e)介观成像系统[35]和(f)台式化显微成像系统[128]用于头部固定小鼠的活体成像;(g)头戴式微型显微镜[129]和(h)光学纤维镜[130]用于自由运动的小鼠活体成像](/richHtml/zgjg/2023/50/15/1507301/img_08.jpg)
图 5. 不同面向活体小鼠的光学成像(或记录)与光遗传神经调控的方法示意图与实物照片。(a)标准的台式化显微成像与光遗传调控方法;(b)植入GRIN透镜用于深层脑区成像或植入微棱镜用于内测脑区成像;用于自由运动动物活体成像的(c)小型化头戴式微型显微镜系统及(d)光纤视镜和光纤光度记录系统;(e)介观成像系统[35]和(f)台式化显微成像系统[128]用于头部固定小鼠的活体成像;(g)头戴式微型显微镜[129]和(h)光学纤维镜[130]用于自由运动的小鼠活体成像
Fig. 5. Schematics and photographs of various approaches for in vivo optical imaging (or recording) and optogenetic neuroregulation in mice. (a) Method of standard benchtop microscopic imaging and optogenetic manipulation; (b) implantable GRIN lens for imaging brain area at depth or implantable microprism for imaging internal brain area; (c) miniaturized head-mounted microscope system and (d) fiberscope and fiber photometry system for in vivo imaging of free-behaving animals; (e) mesoscopic imaging system[35] and (f) benchtop microscopic imaging system[128] for in vivo imaging of head-fixed mouse; (g) head-mounted microscope[129] and (h) fiberscope[130] for in vivo imaging of free-behaving mouse
在激发光源的选择方面,可以分为单光子和多光子激发两种方式。单光子激发的优点在于可以使用相对便宜的二极管泵浦固态激光器(DPSSL)、发光二极管(LED)甚至是白光源(如氙灯或卤钨灯)作为激发光源,而且几乎所有荧光探针的单光子吸收截面都较高,因此较易实现快速、大规模脑成像,如介观尺度甚至是宏观尺度高分辨成像,最新的技术能以亚细胞分辨率对百万量级的神经元进行高动态体积记录[131-133]。通常宽场单光子激发方式的横向分辨率有限且不具备深度分辨能力,适用于大范围脑皮层神经活动研究[
第一台双光子显微镜诞生于1990年[147],当时是以染料飞秒激光器作为激发光源,与其几乎同时期诞生并快速发展的钛宝石固态飞秒激光技术促进了多光子显微镜的快速商用化,如今它已成为活体生物成像与神经科学研究的最有力的工具之一。相比单光子激发方式,多光子成像具有固有的光学层析能力、更高的穿透深度、较低的光毒性等优势,但需使用价格昂贵的飞秒激光光源。传统的扫描式双光子显微镜在进行活体脑组织成像时,视场一般不超过1 mm2,穿透深度为500 μm左右,无法满足大规模、跨脑区、高动态神经记录的应用需求。研究人员一直致力于荧光探针、激发光源、成像器件、原理与算法等方面的改进与革新,试图突破传统双光子显微镜的空间带宽积、成像速度和穿透深度的限制。例如,采用跨区域时间/空间多路复用、时空聚焦、点扩散函数设计等实现大视场快速扫描;通过近红外荧光探针、三光子成像、自适应光学方法、植入GRIN透镜等提高成像深度[122]。为了进一步满足无限制行为的小动物高分辨率成像的研究需求,研究人员成功开发了微型双光子[142,145,148-149]和微型三光子显微镜[150]。相比于单光子微型显微镜,它们具有更高的空间分辨率和更大的成像深度,但由于飞秒激光光源很难像单光子激发光源一样集成到头戴系统上,还需要解决飞秒激光的光纤传输、高速微机电系统扫描、荧光信号的光纤收集等诸多难题,并考虑头戴系统的体积、重量、稳定性等因素的影响。
光纤内窥镜也被引入到头戴式微型显微成像中,它仅通过光纤束或多芯光纤连接台式显微镜和GRIN透镜,因而体积更小、重量更轻,对动物的自由行为影响更小[
2.4 植入式电生理记录与刺激工具介绍
1939年,Hodgkin和Huxley[159-160]将直径为100 μm且内部充满海水的玻璃微管插入枪乌贼的神经轴突之中,首次测出了细胞膜内外的电位差并证实了静息电位假说理论;此外,他们还记录到了膜电位变化并阐明了神经元动作电位的产生机制,这也是人类第一次记录到神经电信号,从而奠定了电生理学的基础。上述静息电位是神经元未受刺激时细胞膜内外的电位差,通常约为-70 mV,该电位将随着其他神经元的兴奋性和抑制性的输入而波动。膜电位分为局部电位和动作电位,局部电位是细胞受到阈下刺激时的微弱电位,通常不能在细胞膜上远距离传播,不在本文的探讨范围之内。神经元的突触输入引起神经元兴奋而使膜电位升高,达到阈值电位(约为-55 mV)时,神经元将被“触发”,即产生动作电位。动作电位由峰电位和后电位组成,幅度为90~130 mV,持续时间为0.5~2.0 ms,可沿细胞膜传播,动作电位是神经元兴奋和活动的标志,是神经信息编码的基本单元。
早期这种填充电解质的玻璃微管是一种改进的细胞内电极,又叫电压钳,这种记录方式需要电极穿透细胞膜,可测高达100 mV的动作电位。然而,它会改变细胞内的电解液成分,影响动作电位发放频率,并对细胞造成机械损伤,因而无法实现长期记录。1957年,Hubel课题组使用自制的带有亚微米级针尖的钨丝电极成功地在猫的大脑皮层中记录了单神经元信号[11,161]。这种方式不需要穿过细胞膜,能够保持细胞完好并进行较长时间记录,随后被广泛用于检测清醒动物的神经元活动。用于电生理学研究的各种植入式神经电极如
![用于电生理学研究的各种植入式神经电极。(a)金属丝电极的扫描电镜(SEM)图像[162];(b)玻璃微丝电极示意图[163];(c)密西根电极示意图[164];(d)犹他电极阵列照片[165];(e)神经像素电极示意图[166];(f)256通道NeuroGrid柔性电极[167];(g)可用于脊髓刺激的e-Dura神经电极[168];(h)可注射纳米电子网电极[169];(i)荧光染色图片显示类神经电极与鼠脑神经元的集成[170];(j)NET神经电极的显微图片(左)和SEM图像(右)[171];(k)“神经流苏”电极示意图[172];(l)Neuralink神经电极植入鼠脑图片[173];(m)micro-CT扫描显示的1024通道柔性电极植入鼠脑后的三维分布[174]](/richHtml/zgjg/2023/50/15/1507301/img_09.jpg)
图 6. 用于电生理学研究的各种植入式神经电极。(a)金属丝电极的扫描电镜(SEM)图像[162];(b)玻璃微丝电极示意图[163];(c)密西根电极示意图[164];(d)犹他电极阵列照片[165];(e)神经像素电极示意图[166];(f)256通道NeuroGrid柔性电极[167];(g)可用于脊髓刺激的e-Dura神经电极[168];(h)可注射纳米电子网电极[169];(i)荧光染色图片显示类神经电极与鼠脑神经元的集成[170];(j)NET神经电极的显微图片(左)和SEM图像(右)[171];(k)“神经流苏”电极示意图[172];(l)Neuralink神经电极植入鼠脑图片[173];(m)micro-CT扫描显示的1024通道柔性电极植入鼠脑后的三维分布[174]
Fig. 6. Implantable neural electrodes for electrophysiology. (a) Scanning electron microscope (SEM) image of wire electrode[162]; (b) illustration of glass microwire electrode[163]; (c) illustration of Michigan electrode[164]; (d) illustration of Utah electrode array[165]; (e) illustration of neuropixels electrode[166]; (f) 256-channel NeuroGrid flexible electrode[167]; (g) e-Dura neural electrodes for stimulation of spinal cord[168]; (h) injectable nanoelectronic mesh electrodes[169]; (i) fluorescent staining image indicating integration of neuron-like electrodes with neurons in mouse brain[170]; (j) micrograph (left) and SEM image (right) of NET neural electrode[171]; (k) illustration of neurotassel electrodes[172]; (l) Neuralink neural electrode implanted in mouse brain[173]; (m) 3D distribution of 1024-channel flexible electrodes implanted in mouse brain shown by micro-CT scan[174]
关于电刺激的研究可以追溯到18世纪中后期,Galvani[176]用弯弓形金属结构连接青蛙的脊髓与大腿,首次通过电火花刺激引起蛙腿肌肉收缩。19世纪的一系列早期动物实验随后开创了电疗法。如今电刺激已被广泛应用于临床,其中,脑深部电刺激(DBS)是转化医学中最具里程碑意义的神经调控技术,它能有效缓解帕金森病、癫痫和肌张力障碍等神经系统病症,具有广泛的临床应用[6-10]。电刺激是将微电极植入到生物组织中,通过电流或电压调节神经元活动和放电模式的过程。大多数金属电极通过在电极表面上产生电子来注入电荷,但在生理系统中,电荷由电解质(离子)携带。在电极-电解质界面,电荷必须通过法拉第(电化学反应)或电容(双层充电)机制从电子转移到离子,通常要求电极材料具有良好的电化学稳定性和耐腐蚀性。与光学方法相比,电刺激会激发相邻神经元,对特定区域的局部刺激几乎是不可能的,其空间分辨率较差且缺乏选择性,提高电刺激的空间分辨率和实现空间特异性是该领域未来努力的方向之一[22,177]。
随着先进半导体微纳加工技术和材料科学的进步,植入式神经微电极的结构和功能不断发展,实现了从单根电极到微电极阵列的提升,包括四极管电极[178]、犹他阵列[165]、密歇根探针[164,179]、神经像素电极[166,180]等[
除了杨氏模量在1~100 GPa范围内作为神经接口的传统电极材料外,一些新兴的且与宿主组织杨氏模量(10 kPa~1 MPa)相匹配的材料如聚合物、弹性体和凝胶等软材料,由于固有的柔软性而被引入,已被证明可以提供更软的组织-电极界面,其可抑制金属电极与其接触的组织之间的机械顺应性差异,电极会随组织移动,从而减少神经接口的损伤。例如,Khodagholy等[167]开发了一种基于有机材料的超柔顺且生物相容性良好的NeuroGrid神经电极阵列[
荧光显微成像、光遗传调控、血流动力学成像和在体电生理的结合能解决电生理技术难以与在体的行为学数据相联系及定义特定的细胞类型的问题。在在体电生理记录过程中,可以运用光遗传学方法激活或抑制神经元,研究神经元电活动、神经环路的电生理变化和行为学的相关性。光遗传学和电生理的结合还可以形成闭环模式,例如癫痫患者通常出现神经元放电异常增加,通过电生理记录神经元的电活动,当神经元放电异常增加时,光遗传学的开关被触发,发射出激发光,抑制神经元电活动[93];目前在临床环境中使用DBS治疗时不能区分不同的神经环路,Spix等[183]结合光遗传学开发了一种巧妙的电刺激方案,极大改进了针对特定神经元群体的选择性刺激,并在帕金森病的小鼠模型中产生了长期效果。在行为学过程中,运用电生理记录多基因特异性表达的神经元很困难,但GECI可以在细胞分辨率的基础上长期记录不同基因定义的神经活动,并揭示其与行为学的相关性。目前许多研究往往将GECI、光遗传学和电生理技术结合,研究复杂大脑神经环路机制[184],这对多模态神经接口的系统集成提出了重大挑战。
3 植入式光电神经接口前沿进展
3.1 基于光纤和光波导的神经接口
光纤光度记录法是常用的记录在体神经元群体活动的方法,通常采用标准的平口多模光纤传输激发光与荧光信号,根据收集到的荧光信号强度来表征群体神经活动[185]。除了记录神经元的荧光信号外,光纤在光遗传技术提出后不久就被用于小鼠运动皮层的神经调控[186]。在早期的研究中,光纤只能用于刺激神经元,刺激后的反应需要依靠动物的行为或其他设备进行检测[187];之后有研究小组使用单根光纤实现了光刺激,同时通过荧光信号检测神经元活动[188],但单根光纤不能刺激大范围脑组织;增加光功率虽然可以提高被光照刺激的神经元数量,但会诱发严重的热效应,从而对大脑造成损伤[189]。典型的直径为200 μm的阶跃折射率光纤可以进行体内光遗传学实验的刺激照明和信号读取,光纤束可以通过计算机生成全息图,实现高度空间特异性的深部脑光遗传学刺激以及接近单神经元分辨率的荧光成像[190]。由于植入光纤体积小且方便同时记录多个大脑区域的信号,因此可将光遗传学与光纤记录结合,使研究人员能够研究特定细胞的投射关系和功能意义;而将光纤刺激和在体电生理结合,可以形成闭环模式,例如在在体电生理记录过程中,运用光遗传学方式激活或抑制神经元,研究神经元电活动、环路的电生理变化和行为学的相关性[191];此外,将fMRI技术与光纤光度记录结合,可以使fMRI扫描具有细胞分辨率,验证星形胶质细胞Ca2+水平的变化与BOLD信号之间的关联,并阐明单个神经元亚群对大脑神经环路的贡献[192]。
光纤的直径是影响植入损伤的关键参数,例如植入直径为数百微米的光纤会导致过多神经元损伤,而直径较小的光纤又增加了其尖端的热效应,因此在选择光纤时需要在其直径和热效应之间进行权衡。在光纤材料选择方面,二氧化硅材质的光纤已被广泛应用在光遗传学操作中,它们具有化学惰性并且光学损耗低,但与脑组织相比,二氧化硅杨氏模量过高,对自由活动的实验动物可能会造成神经元损伤。纤维素薄膜和水凝胶等柔性光波导材料也被用于光遗传学调控,但其尺寸往往较大且光的传输损耗较高[193]。近期部分研究通过可植入和可生物降解光纤实现了体内深部脑荧光传感和光遗传学操控[194]。平口光纤在大脑内的照明范围难以控制,增大光功率可以提高照明范围,但是也会带来更严重的热效应。Pisanello等[195]通过在熔拉法制作的锥形光纤上镀膜和聚焦离子束(FIB)制造光学窗口,实现了用单根光纤动态刺激不同的大脑区域;此外,他们通过选择光纤输入端的模态子集,实现了不同窗口的可寻址光发射。之后该研究小组使用未在锥面镀膜的锥形光纤,通过远程调控光纤的光输入角度,在锥面上几毫米的范围内改变发光位点,实现了脑组织的大范围照明和动态光照控制[196]。
![植入式光波导与光电极。(a)用于神经接口的锥形光纤示意图[184];(b)脑组织中平口光纤和锥度光纤的荧光收集示意图[197];(c)具有七窗口多点发射的锥度光纤SEM显微图片[195];(d)光子神经探针阵列示意图[195];(e)光子神经探针相控阵E-pixel的SEM图[195];(f)基于可见光谱角度选择单光子雪崩二极管(SPAD)探测器的D-pixel阵列[198];(g)GaN-μLED的彩色扫描电镜图(上)与μLEDs在培养细胞中的荧光图像(下)[199];(h)μLED探针的显微图像和扫描电镜图像[200];(i)集成无线供电的μLED神经探针[201];(j)制备的光极上的尖端的扫描电镜图像[202];(k)光纤拉锥法制备多功能光纤[203];(l)锥形光纤侧面制备的金属电极的设计图(上)和扫描电镜图像(下)[204]](/richHtml/zgjg/2023/50/15/1507301/img_10.jpg)
图 7. 植入式光波导与光电极。(a)用于神经接口的锥形光纤示意图[184];(b)脑组织中平口光纤和锥度光纤的荧光收集示意图[197];(c)具有七窗口多点发射的锥度光纤SEM显微图片[195];(d)光子神经探针阵列示意图[195];(e)光子神经探针相控阵E-pixel的SEM图[195];(f)基于可见光谱角度选择单光子雪崩二极管(SPAD)探测器的D-pixel阵列[198];(g)GaN-μLED的彩色扫描电镜图(上)与μLEDs在培养细胞中的荧光图像(下)[199];(h)μLED探针的显微图像和扫描电镜图像[200];(i)集成无线供电的μLED神经探针[201];(j)制备的光极上的尖端的扫描电镜图像[202];(k)光纤拉锥法制备多功能光纤[203];(l)锥形光纤侧面制备的金属电极的设计图(上)和扫描电镜图像(下)[204]
Fig. 7. Implanted optical waveguides and optical electrodes. (a) Schematics of tapered fibers for neural interfaces[184]; (b) schematic of fluorescence collection of flat-ended and tapered fibers implanted in brain tissue[197]; (c) SEM micrograph of tapered fiber with seven-window multipoint emission[195]; (d) schematic of photonic neural probe array[195]; (e) SEM image of phased array E-pixel of photonic neural probe[195]; (f) D-pixels based on visible spectrum angle-selective single-photon avalanche diode (SPAD) detectors[198]; (g) color SEM image of GaN-μLED (top) and fluorescence image of μLEDs in cultured cells(bottom)[199]; (h) microscopic image and SEM image of μLED probe[200]; (i) integrated wireless powered μLED neural probe[201]; (j) SEM image of tip on prepared optrode[202]; (k) fabrication of multifunctional fiber by fiber tapering method[203]; (l) design drawing (top) and SEM image (bottom) of fabricated metal electrode on side of tapered fiber[204]
为了减小植入式光极的体积并增加通道数,研究人员基于微机电系统(MEMS)工艺制备的光波导器件,展示出了集成器件的优点:与直径在100 μm以上的光纤相比,光波导采用平板结构,截面尺寸只有几十微米,厚度在10 μm以下,大大减小了植入损伤。相比于光纤,波导在材料尺寸、通路及出光方向等方面的可选择性多,在设计和应用方面具有很大灵活性。为了克服自由空间和内窥镜功能性成像的局限性,Moreaux等[198]构思了一种名为集成光子神经探针的方法[
基于光波导的光电极需要光源和波导器件耦合,但耦合效率较低,而微像素发光二极管(μLED)具有体积小、生物兼容性良好的特点,可以植入神经组织作为微型光源调控动物的神经活动[199,205-209]。例如,μLED可以集成到植入式神经探针的工作端,用于直接刺激组织或与设备上的波导耦合,如
3.2 基于柔性电极的多模态神经接口
用于记录和刺激神经元活动的光学方法能够以亚细胞的分辨率来研究大规模、多维度神经网络。光学工具与电生理学记录的结合可以充分发挥光学成像的高空间分辨率以及电生理学的高时间分辨率和保真度,对大脑神经环路进行全面的多尺度研究。然而,传统的不透明金属微电极会遮挡光学成像且产生光电伪影,无法有效进行多模态神经科学研究。
传统的神经植入器件大多基于金属或硅材质,一般尺寸较大且不透光,这种非透明植入物会减少光的传输和阻挡光学成像。随着微纳制造技术和材料科学的进步,更小尺寸、更高功能密度的神经接口已经实现,因此可以通过电极材料的几何结构设计来构筑透明神经接口,例如可以将电极设计成网格状和金属纳米线等结构[171,212],金属导线的间距越宽网格越大,神经接口的透明度越高。此外,还可以减小电极的尺寸以减小光学遮挡物的面积,进而提高神经接口的透明度。其挑战在于电极尺寸的减小会增大神经记录位点的电化学阻抗,从而降低信噪比,可以通过电化学镀或提高电极的表面积来达到增加导电性和透明度的目的[213]。在光学和电生理学集成应用的系统构架上,基于柔性衬底的神经探针的优势也是明显的。
![光电多模态神经接口示意图与应用实例。(a)使用刚性神经电极对小鼠同时进行活体光学成像和电记录时的颅窗设置示意图;使用电极对转基因小鼠进行(b)电刺激前和(c)刺激后的钙成像结果[214];(d)使用柔性神经电极对小鼠同时进行活体光学成像和电记录时的颅窗设置示意图;(e)植入小鼠皮层2个月后探针-组织界面及探针的细胞和血管结构的活体双光子成像3D重建[171];(f)植入2个月后在NET-e探针周围的Thy1-YFP小鼠体内双光子神经元图像的3D重建[215];对小鼠脑皮层(g)同时进行双光子扫描血流成像和柔性电极记录[128]以及(h)同时进行激光散斑衬度血流成像和柔性电极记录[35];(i)宽视场钙成像结合电生理同步记录小鼠皮层和海马区的神经信号[216];(j)头戴式微型显微镜结合刚性神经电极同时对小鼠进行成像和电刺激示意图(左)和实物图(右)[143];(k)头戴式显微镜结合柔性神经电极构成的多模态神经接口示意图(左)和实物图(右)[217]](/richHtml/zgjg/2023/50/15/1507301/img_11.jpg)
图 8. 光电多模态神经接口示意图与应用实例。(a)使用刚性神经电极对小鼠同时进行活体光学成像和电记录时的颅窗设置示意图;使用电极对转基因小鼠进行(b)电刺激前和(c)刺激后的钙成像结果[214];(d)使用柔性神经电极对小鼠同时进行活体光学成像和电记录时的颅窗设置示意图;(e)植入小鼠皮层2个月后探针-组织界面及探针的细胞和血管结构的活体双光子成像3D重建[171];(f)植入2个月后在NET-e探针周围的Thy1-YFP小鼠体内双光子神经元图像的3D重建[215];对小鼠脑皮层(g)同时进行双光子扫描血流成像和柔性电极记录[128]以及(h)同时进行激光散斑衬度血流成像和柔性电极记录[35];(i)宽视场钙成像结合电生理同步记录小鼠皮层和海马区的神经信号[216];(j)头戴式微型显微镜结合刚性神经电极同时对小鼠进行成像和电刺激示意图(左)和实物图(右)[143];(k)头戴式显微镜结合柔性神经电极构成的多模态神经接口示意图(左)和实物图(右)[217]
Fig. 8. Schematics and applications of optoelectronic multimodal neural interface. (a) Cranial window setup in mouse for simultaneous in vivo optical imaging and electrical recording using rigid neural probe; calcium imaging results of transgenic mice using electrodes (b) before and (c) after electrical stimulation[214]; (d) cranial window setup in mouse for simultaneous in vivo optical imaging and electrical recording using flexible neural probe; (e) 3D reconstruction of in vivo two-photon image of cellular and vascular structures at probe-tissue interface and probe after implantation for two months in mouse cortex[171]; (f) 3D reconstruction of in vivo two-photon neuron image in Thy1-YFP mouse surrounding NET-e probe after implantation for two months[215]; (g) simultaneous two-photon scanning blood flow imaging and flexible electrode recording of mouse cortex[128], and (h) simultaneous laser speckle contrast blood flow imaging and flexible electrode recording[35]; (i) neural signal recording by simultaneous wide-field calcium imaging and electrophysiology in mouse cortex and hippocampus[216]; (j) schematic (left) and application (right) of simultaneous imaging and electrical stimulation in mouse by combination of head-mounted miniature microscope and rigid neural electrodes[143]; (k) schematic (left) and application (right) of multimodal neural interface constructed by head-mounted microscope combined with flexible neural electrodes[217]
在进行高时空精度神经记录,特别是对神经血管微环境进行连续稳定追踪时,保持神经接口附近的神经血管组织活性尤为重要。如2.4节所述,传统的神经植入器件具有大而刚性的非生物特征,这种机械差异会形成剪切力,并在组织运动和电极微位移期间引发慢性炎症等生物反应,很难与生物组织集成为长期稳定的神经接口[214]。最近发展起来的超柔神经电极技术有效缓解了这一难题[20,35,171,174][
此外,为了构建多模态神经接口用以有效研究自由运动小鼠行为学与神经活动的映射,基于头戴式微型显微镜和神经电极的集成系统也已被开发出来[143,217,224]。
3.3 植入式透明神经接口
传统金属材质的电极除会引起光学遮挡外,在进行激光扫描成像应用研究时,还会产生光电伪影,这是由于光照射到金属记录点时产生了光伏效应,也称贝克勒尔效应。这种光激励电流或电压所引起的电极记录伪迹严重降低了神经信号的信噪比,一般可以通过降低电极界面的阻抗、采用对光不敏感的电极材料、作好激发光和记录电极点及引线之间的隔离等多种方法来提高记录信号的信噪比,从而系统能记录到更多的神经元活动、更准确反映光刺激效果[37]。神经接口材料和制造工艺的发展使得透明微电极阵列制备成为可能,它可在同一大脑位置同时进行无伪影成像和记录。这些透明微电极的记录位点大多为透明导电材料,绝缘层一般为薄而柔韧的聚合物薄膜,例如聚对二甲苯、聚酰亚胺(PI)、聚对苯二甲酸乙二醇酯(PET)等。这种植入式透明神经接口可以将成像的高光学透明度与神经记录所需的高电导率结合,为神经科学研究和临床应用提供了新的范式,如
![基于透明神经电极的多模态神经接口示意图与应用实例。(a)透明神经电极与不透明神经电极的比较[225];利用(b)不透明铂金电极阵列和(c)透明石墨烯电极阵列对小鼠大脑皮层进行OCT[225];(d)透明的石墨烯电极阵列的电记录和刺激与同时记录的FITC-Dextran绿色荧光染料标记血管的荧光显微镜图像[225];(e)用石墨烯微电极阵列曝光的明场显微成像[226];(f)同时电生理记录和光学成像示意图[227];(g)石墨烯电极阵列置于小鼠皮层的图像[227];(h)位于小鼠皮层的石墨烯电极阵列的相对位置和4-AP药物注射的位置[227];(i)归一化的荧光强度[227];(j)µECoG记录信号[227]](/richHtml/zgjg/2023/50/15/1507301/img_12.jpg)
图 9. 基于透明神经电极的多模态神经接口示意图与应用实例。(a)透明神经电极与不透明神经电极的比较[225];利用(b)不透明铂金电极阵列和(c)透明石墨烯电极阵列对小鼠大脑皮层进行OCT[225];(d)透明的石墨烯电极阵列的电记录和刺激与同时记录的FITC-Dextran绿色荧光染料标记血管的荧光显微镜图像[225];(e)用石墨烯微电极阵列曝光的明场显微成像[226];(f)同时电生理记录和光学成像示意图[227];(g)石墨烯电极阵列置于小鼠皮层的图像[227];(h)位于小鼠皮层的石墨烯电极阵列的相对位置和4-AP药物注射的位置[227];(i)归一化的荧光强度[227];(j)µECoG记录信号[227]
Fig. 9. Schematics and applications of multimodal neural interface based on transparent neural electrode. (a) Comparison between transparent neural electrodes and opaque neural electrodes[225]; OCT with (b) opaque platinum electrode array and (c) transparent graphene electrode array in mouse cortex[225]; (d) electrical recording and stimulation with transparent graphene electrode array and simultaneously recorded fluorescence microscope image of FITC-Dextran green fluorescent dye labeled blood vessels[225]; (e) bright-field microscope imaging exposed with graphene microelectrode array[226]; (f) schematic of simultaneous electrophysiological recording and optical imaging[227]; (g) image of graphene electrode array implanted in mouse cortex[227]; (h) relative positions of graphene electrode array in mouse cortex and location of 4-AP drug injection[227]; (i) normalized fluorescence intensity[227]; (j) μECoG recording signals[227]
以石墨烯材料为例,它是一种近年来兴起的纳米材料,具有出色的光学、机械和化学稳定性及良好的导电性、生物相容性,特别是单层石墨烯在紫外和红外光波段具有>97%的透射率,在光遗传调控和荧光显微成像方面具有极大优势,目前已被广泛应用于透明神经接口制备与多功能集成领域[225-226,228-230]。石墨烯的光激发系数较低,在光学成像与调控特性上优于传统金属电极,研究者通过使用其宽波段透明度,验证了钙成像和电生理信号之间的相似性。
4 多模态神经数据分析
复杂的神经活动可在大脑的不同尺度上对与行为和认知相关的丰富信息进行编码,这一尺度范围涵盖了单个神经元到不同大脑脑区。然而,大部分研究通常集中在单一模式获取的神经活动上,并在单一尺度上使用同种模态的记录对神经动力学进行建模和解码,这些研究往往被限制在同一时空水平内的神经动力学上。这些传统研究方法因为其模态的单一性,对神经活动的评估是不完全的。逐步发展起来的多种神经技术——电学、光学、化学等方法使我们能够在不同的时间和空间尺度上记录和扰动神经活动。神经技术的不断突破促使神经科学研究的范式不断转变,以便更加有效分析多模态和多尺度的神经元活动[235-236]。多模态神经数据分析可以综合考虑空间和时间分辨率,并在多个尺度和维度上整合和阐明神经信息,针对大脑认知、决策等神经活动的复杂机制提供更加全面深入的理解,这对于开发有效的临床治疗方法至关重要。鉴于目前多模态数据分析尚无确定范式,我们将分别阐述光学、电学以及行为学数据分析的一般方法。
随着功能光学成像和基因编码荧光指示技术的发展,在体神经活动成像的时空分辨率、视场和深度等不断取得突破,例如双光子显微镜、微型头戴式显微镜和光纤记录法能实时监测动物大脑的钙信号,图案化的双光子光遗传技术能定制化控制神经元群体,神经科学家有望非常精确地“读取”和“改写”神经活动模式,并能够更好地理解和操控复杂的神经环路,可获取的数据通量不断增大。为了充分发挥巨大的时空数据集的潜力,将神经活动与行为和刺激联系起来,并揭示大脑中的精细神经环路,准确的自动化数据处理变得越来越重要。光学功能成像数据分析的首要问题是识别神经元或群体的空间轮廓和相应的时间轨迹。
![神经成像和神经记录数据分析流程示意图。(a)神经元的光学成像数据示意图[37];(b)神经元光学成像数据的分析流程;(c)神经元的电信号数据示意图[128];(d)神经元电信号数据的分析流程](/richHtml/zgjg/2023/50/15/1507301/img_13.jpg)
图 10. 神经成像和神经记录数据分析流程示意图。(a)神经元的光学成像数据示意图[37];(b)神经元光学成像数据的分析流程;(c)神经元的电信号数据示意图[128];(d)神经元电信号数据的分析流程
Fig. 10. Schematics of neuroimaging and neural recording data analysis process. (a) Schematic of optical imaging data of neurons[37]; (b) analysis process for optical imaging data of neurons; (c) schematic of electrical signal data of neurons[128]; (d) analysis process for electrical signal data of neurons
在电生理方法上,研究者通常使用植入式神经电极来记录神经元的单位活动(SUA)或多单位活动(MUA),这些活动反映了大脑中一个或少量神经元的动作电位(“峰电位”),可用于确定神经元放电速率与行为变量共同变化的规律。动作电位属于高频信号,一般采用20 kHz以上的高速采样频率进行采集和记录。根据记录到的神经元胞外动作电位波形,运用主成分分析(PCA)技术等机器学习特征提取聚类分析方法,可对记录电极周围不同空间位置的神经元放电信号进行甄别,从而获得较精确的单神经元放电时间序列[
与周围环境进行物理交互会触发大量神经系统中的神经活动,因此理解运动与大脑功能的关系需要进行高精度时空量化。行为是大脑中潜在神经计算的最重要输出,它不仅复杂,而且是多维度且高度依赖于环境的,利用行为的定量描述可以将大脑活动与动物运动联系起来,进而解读神经环路、认知过程和行为之间的关系。计算机视觉的最新进展使得对动物行为进行准确、快速测量成为现实,并已经取得了显著的成果,包括DeepLabCut等基于深度学习的软件包能将任何包含运动的视频转换成动作捕捉信息,对动物的运动实现基于视频的跟踪,为行为表征提供了巨大的数据库[252-254]。动作捕捉在理疗和康复领域也已应用多时,例如医生通过对行为进行主观判断,能诊断自闭症或评估运动功能的恢复状况,将来基于视频的运动分析可能成为所有运动障碍患者的首要诊断步骤[255]。应用不同模态与不同行为范式可以在全脑尺度下揭示复杂任务中神经活动的动态变化,如提供更全面的神经功能图景、克服时空分辨率的冲突、量化尺度和数据类型之间的耦合、降低对电子硬件和计算能力的要求、延长设备的使用寿命、提供不同神经模式间的连接性或因果关系并提高解码性能等。
5 总结与展望
从18世纪Galvani用金属工具对蛙腿神经进行电刺激建立“动物电学说”,到19世纪Cajal通过光学显微观测奠定“神经元学说”,再到20世纪细胞内电极、电压钳、膜片钳、功能核磁共振、基因编码荧光蛋白等系列神经工具的发明及21世纪初光遗传学技术的诞生,神经科学的每一次重大突破都离不开神经技术与工具的革新。大脑是宇宙间最复杂的物体和人类最重要的器官,理解大脑的结构与功能是当今最具挑战性的前沿科学问题,了解大脑神经系统如何运作是定量和具体描述诸如行为和认知缺陷神经疾病的必要前提,理解和破译拥有近1000亿个神经元和100万亿个神经连接的人类大脑是科学和工程领域面临的最大挑战之一。从理论上讲,同时记录哺乳动物全脑神经元活动是可以实现的[256]。随着神经技术的突飞猛进[21,43,145,174,180,257-273],神经记录领域的“摩尔定律”已经变得不再适用(
![神经信号记录规模的增长趋势[21,43,145,174,180,257-273]](/richHtml/zgjg/2023/50/15/1507301/img_14.jpg)
图 11. 神经信号记录规模的增长趋势[21,43,145,174,180,257-273]
Fig. 11. Increasing trend of neural signal recording scale[21,43,145,174,180,257-273]
荧光蛋白和光遗传学工具的使用已经彻底改变了神经科学,对神经元特异性标记和操控的需求表明该领域将会持续高速发展。研究者希望下一代 GECI将在速度和灵敏度之间取得更好的平衡,同时期待能进一步扩展GECI的种类和光谱范围,能有更好的计算方法来推断Ca2+的峰值序列活动[242];对于GEVI,则希望具有更少静息荧光和更大动态范围的高亮度,能在降低照明光强的同时保持高保真峰值,从而延长成像时间。此外,大多数相机和双光子显微镜仍然缺乏分辨单个动作电位所需的千赫兹图像帧采集速率,光学仪器的进步才能使电压成像完全实现;光遗传学工具的设计和数据解读必须综合考虑各类限制因素,例如视蛋白动力学特征、光谱重叠较大、光激活/诱导效率不高、神经递质的自发释放等系列问题,需要充分发挥遗传学手段优势,进行视蛋白工程结构学上的调整,并将光遗传学与其他神经方法和工具充分结合,持续助力神经环路与行为学关联研究。随着遗传工具和疾病模型在非人灵长类中的发展[275],非人灵长类的定制化成像方法对于研究大脑功能和直接影响人类健康的功能障碍具有重要意义。
虽然神经电极材料和制备工艺愈发先进,但仍有较多提升空间。例如水凝胶的杨氏模量几乎与神经组织相当,基于水凝胶的神经接口因其固有的柔软性和与生物组织的化学相容性而引起了广泛的兴趣;细胞外基质蛋白、肽、脂质和多糖等天然材料具有优异的生物相容性,已成为提高神经接口性能的有力候选者;受生物学启发,神经探针表面的功能化处理,如细胞附着分子、抗炎涂层和神经营养因子等[276],已被普遍用于改善神经电极的排异反应,或使神经元更靠近电极以增强组织接受度;可生物降解的神经接口可能是临床上避免二次手术的另一种解决方案[277],未来需要充分评估和确定基于这些材质的神经接口的力学、电子和生化特性及其与生物组织的相互作用。
大多数神经调控使用开环方法,然而由于生物个体差异、解剖结构的多样性以及神经系统的动态变化特性,神经调控方案需要不断调整。因此,使用能够连续、稳定监测感兴趣区域的活动并能实时处理和刺激大脑神经元的闭环系统可以实现更加准确和有针对性的治疗[278]。当前的神经接口通常依赖有线系统进行数据传输和供电,不仅尺寸过大、易受外部损坏,还会抑制动物的自由行为并导致更多的免疫反应,从而降低了实验方案的适用性以及行为学结果的可靠性。将无线系统集成到神经接口可以提高系统的整体稳定性。目前其应用范围已从鼠类扩大到鸟类、鱼类和非人灵长类动物等,其尺寸、性能、协同集成和能耗仍有待进一步优化[279-281]。
多模态神经数据分析方面面临的主要挑战是建立多尺度建模和分析方法,为理解神经活动、疾病机理和诊疗、认知及行为提供多功能支持,还需要建立跨越模态的神经活动操控方案,对多模态同步记录神经活动进行分析,从而理解大脑各个尺度的神经元活动与思想行为的映射关系。其次,缺乏用于分析在不同空间和时间尺度上运行的高维数据集的既定框架,例如缺少神经元活动的多模态光学和电生理学映射中出现的数据集[22],以及在长期研究中收集高频、多通道和多模态数据会进一步加剧数据收集带宽和存储问题。最后,缺乏标准化的实验设计和实验报告降低了数据集之间的比较性和可重复性,需要创建能以标准化格式共享数据的平台[282]。
值得一提的是,我国科学家在神经接口的关键方法与工具方面也取得了系列成果。例如清华大学在大视场高分辨成像仪器、计算成像方法等方面[131,139-140,283-284],北京大学在微型双光子显微镜[142,145,149]、基因编码荧光探针等方面[285-290],中国科学院各研究所在神经电极研制与应用等方面均有所突破。脑科学研究方兴未艾,我国于2021年正式启动“中国脑计划”,已对脑科学与类脑研究作出重点部署,而美国脑计划已迭代至2.0版本[291]。可以预见,未来随着神经探针结构和材料的发展以及纳米粒子、染料分子和基因编码蛋白等合成技术的革新,神经接口技术将不断突破神经记录和刺激寿命、定位和特异性的极限,最终打破生物活体组织和物理设备工具之间的边界。这些融合了先进光学与微纳电子技术、光遗传学、神经活动指示剂、声学和磁学等技术的神经方法和工具,为实现新的多模态神经信息交互提供了前所未有的机会,它们将具备空前的神经记录和调控能力,为大脑活动的多模态操控提供一个强大的范式,甚至有望从根本上改变大脑活动与物理世界的映射关系。
[1] Insel T R, Landis S C, Collins F S. The NIH Brain initiative[J]. Science, 2013, 340(6133): 687-688.
[2] Markram H. The blue brain project[J]. Nature Reviews Neuroscience, 2006, 7(2): 153-160.
[3] Rajasethupathy P, Ferenczi E, Deisseroth K. Targeting neural circuits[J]. Cell, 2016, 165(3): 524-534.
[4] Deep-Brain Stimulation for Parkinson's Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease[J]. The New England Journal of Medicine, 2001, 345(13): 956-963.
[5] Gooch C L, Pracht E, Borenstein A R. The burden of neurological disease in the United States: a summary report and call to action[J]. Annals of Neurology, 2017, 81(4): 479-484.
[6] Perlmutter J S, Mink J W. Deep brain stimulation[J]. Annual Review of Neuroscience, 2006, 29: 229-257.
[7] Kringelbach M L, Jenkinson N, Owen S L F, et al. Translational principles of deep brain stimulation[J]. Nature Reviews Neuroscience, 2007, 8(8): 623-635.
[8] Mayberg H S, Lozano A M, Voon V, et al. Deep brain stimulation for treatment-resistant depression[J]. Neuron, 2005, 45(5): 651-660.
[9] Lozano A M, Lipsman N, Bergman H, et al. Deep brain stimulation: current challenges and future directions[J]. Nature Reviews Neurology, 2019, 15(3): 148-160.
[10] Krauss J K, Lipsman N, Aziz T, et al. Technology of deep brain stimulation: current status and future directions[J]. Nature Reviews Neurology, 2021, 17(2): 75-87.
[11] KandelE R, SchwartzJ H, JessellT M, et al. Principles of neural science[M]. 4th ed. New York: McGraw-Hill, 2000.
[12] Hong G S, Lieber C M. Novel electrode technologies for neural recordings[J]. Nature Reviews Neuroscience, 2019, 20(6): 330-345.
[16] Someya T, Bao Z N, Malliaras G G. The rise of plastic bioelectronics[J]. Nature, 2016, 540(7633): 379-385.
[17] Chen R, Canales A, Anikeeva P. Neural recording and modulation technologies[J]. Nature Reviews Materials, 2017, 2(2): 1-16.
[18] Patel S R, Lieber C M. Precision electronic medicine in the brain[J]. Nature Biotechnology, 2019, 37(9): 1007-1012.
[19] Song E M, Li J H, Won S M, et al. Materials for flexible bioelectronic systems as chronic neural interfaces[J]. Nature Materials, 2020, 19(6): 590-603.
[20] He F, Lycke R, Ganji M, et al. Ultraflexible neural electrodes for long-lasting intracortical recording[J]. iScience, 2020, 23(8): 101387.
[21] Urai A E, Doiron B, Leifer A M, et al. Large-scale neural recordings call for new insights to link brain and behavior[J]. Nature Neuroscience, 2022, 25(1): 11-19.
[22] Rivnay J, Wang H L, Fenno L, et al. Next-generation probes, particles, and proteins for neural interfacing[J]. Science Advances, 2017, 3(6): e1601649.
[23] Frank J A, Antonini M J, Anikeeva P. Next-generation interfaces for studying neural function[J]. Nature Biotechnology, 2019, 37(9): 1013-1023.
[24] Feiner R, Dvir T. Tissue-electronics interfaces: from implantable devices to engineered tissues[J]. Nature Reviews Materials, 2018, 3(1): 1-16.
[25] Salatino J W, Ludwig K A, Kozai T D Y, et al. Glial responses to implanted electrodes in the brain[J]. Nature Biomedical Engineering, 2017, 1(11): 862-877.
[26] Vázquez-Guardado A, Yang Y Y, Bandodkar A J, et al. Recent advances in neurotechnologies with broad potential for neuroscience research[J]. Nature Neuroscience, 2020, 23(12): 1522-1536.
[27] Tian H H, Xu K, Zou L, et al. Multimodal neural probes for combined optogenetics and electrophysiology[J]. iScience, 2022, 25(1): 103612.
[28] 方英. 神经界面[J]. 物理化学学报, 2020, 36(12): 2009081.
Fang Y. Neural interfaces[J]. Acta Physico-Chimica Sinica, 2020, 36(12): 2009081.
[29] 刘杨, 段小洁. 基于碳纳米材料的神经电极技术[J]. 物理化学学报, 2020, 36(12): 2007066.
Liu Y, Duan X J. Carbon-based nanomaterials for neural electrode technology[J]. Acta Physico-Chimica Sinica, 2020, 36(12): 2007066.
[30] 史钊, 李丽珠, 赵钰, 等. 植入式生物医疗光电子器件与系统[J]. 中国激光, 2018, 45(2): 0207001.
[31] 王一帆, 郑瑶, 朱玥, 等. 精准光遗传学的关键技术及进展[J]. 激光与光电子学进展, 2022, 59(8): 0800001.
[32] Liu R, Li Z Y, Marvin J S, et al. Direct wavefront sensing enables functional imaging of infragranular axons and spines[J]. Nature Methods, 2019, 16(7): 615-618.
[33] Adesnik H, Abdeladim L. Probing neural codes with two-photon holographic optogenetics[J]. Nature Neuroscience, 2021, 24(10): 1356-1366.
[34] Zhang Z H, Russell L E, Packer A M, et al. Closed-loop all-optical interrogation of neural circuits in vivo[J]. Nature Methods, 2018, 15(12): 1037-1040.
[35] He F, Sullender C T, Zhu H L, et al. Multimodal mapping of neural activity and cerebral blood flow reveals long-lasting neurovascular dissociations after small-scale strokes[J]. Science Advances, 2020, 6(21): eaba1933.
[36] Zou L, Tian H H, Guan S L, et al. Self-assembled multifunctional neural probes for precise integration of optogenetics and electrophysiology[J]. Nature Communications, 2021, 12(1): 1-9.
[37] Grienberger C, Giovannucci A, Zeiger W, et al. Two-photon calcium imaging of neuronal activity[J]. Nature Reviews Methods Primers, 2022, 2(1): 67.
[38] Cho Y U, Lim S L, Hong J H, et al. Transparent neural implantable devices: a comprehensive review of challenges and progress[J]. Npj Flexible Electronics, 2022, 6(1): 1-18.
[39] Park Y, Park S Y, Eom K. Current review of optical neural interfaces for clinical applications[J]. Micromachines, 2021, 12(8): 925.
[40] Cohen L B, Salzberg B M, Grinvald A. Optical methods for monitoring neuron activity[J]. Annual Review of Neuroscience, 1978, 1: 171-182.
[41] Abdelfattah A S, Kawashima T, Singh A, et al. Bright and photostable chemigenetic indicators for extended in vivo voltage imaging[J]. Science, 2019, 365(6454): 699-704.
[42] Piatkevich K D, Bensussen S, Tseng H A, et al. Population imaging of neural activity in awake behaving mice[J]. Nature, 2019, 574(7778): 413-417.
[43] Villette V, Chavarha M, Dimov I K, et al. Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice[J]. Cell, 2019, 179(7): 1590-1608.
[44] Kannan M, Vasan G, Huang C, et al. Fast, in vivo voltage imaging using a red fluorescent indicator[J]. Nature Methods, 2018, 15(12): 1108-1116.
[45] Inoue M. Genetically encoded calcium indicators to probe complex brain circuit dynamics in vivo[J]. Neuroscience Research, 2021, 169: 2-8.
[46] Barson D, Hamodi A S, Shen X L, et al. Simultaneous mesoscopic and two-photon imaging of neuronal activity in cortical circuits[J]. Nature Methods, 2020, 17(1): 107-113.
[47] Lohr C, Beiersdorfer A, Fischer T, et al. Using genetically encoded calcium indicators to study astrocyte physiology: a field guide[J]. Frontiers in Cellular Neuroscience, 2021, 15: 690147.
[48] Stepnoski R A, LaPorta A, Raccuia-Behling F, et al. Noninvasive detection of changes in membrane potential in cultured neurons by light scattering[J]. Proceedings of the National Academy of Sciences of the United States of America, 1991, 88(21): 9382-9386.
[49] Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application[J]. NeuroImage, 2012, 63(2): 921-935.
[50] Wu Z F, Lin D Y, Li Y L. Pushing the frontiers: tools for monitoring neurotransmitters and neuromodulators[J]. Nature Reviews Neuroscience, 2022, 23(5): 257-274.
[51] Choe M, Titov D V. Genetically encoded tools for measuring and manipulating metabolism[J]. Nature Chemical Biology, 2022, 18(5): 451-460.
[52] Day-Cooney J, Dalangin R, Zhong H N, et al. Genetically encoded fluorescent sensors for imaging neuronal dynamics in vivo[J]. Journal of Neurochemistry, 2023, 164(3): 284-308.
[53] Dong C Y, Zheng Y, Long-Iyer K, et al. Fluorescence imaging of neural activity, neurochemical dynamics, and drug-specific receptor conformation with genetically encoded sensors[J]. Annual Review of Neuroscience, 2022, 45: 273-294.
[54] Berridge M J, Bootman M D, Roderick H L. Calcium signalling: dynamics, homeostasis and remodelling[J]. Nature Reviews Molecular Cell Biology, 2003, 4(7): 517-529.
[55] Grynkiewicz G, Poenie M, Tsien R Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties[J]. Journal of Biological Chemistry, 1985, 260(6): 3440-3450.
[56] Tsien R Y. A non-disruptive technique for loading calcium buffers and indicators into cells[J]. Nature, 1981, 290(5806): 527-528.
[57] Ohki K, Chung S, Ch'ng Y H, et al. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex[J]. Nature, 2005, 433(7026): 597-603.
[58] Takano T, Han X N, Deane R, et al. Two-photon imaging of astrocytic Ca2+ signaling and the microvasculature in experimental mice models of Alzheimer's disease[J]. Annals of the New York Academy of Sciences, 2007, 1097: 40-50.
[59] Miyawaki A, Llopis J, Heim R, et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin[J]. Nature, 1997, 388(6645): 882-887.
[60] Baird G S, Zacharias D A, Tsien R Y. Circular permutation and receptor insertion within green fluorescent proteins[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(20): 11241-11246.
[61] Tian L, Hires S A, Mao T Y, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators[J]. Nature Methods, 2009, 6(12): 875-881.
[63] Kim T H, Schnitzer M J. Fluorescence imaging of large-scale neural ensemble dynamics[J]. Cell, 2022, 185(1): 9-41.
[64] Zhao Y X, Araki S, Wu J H, et al. An expanded palette of genetically encoded Ca²⁺ indicators[J]. Science, 2011, 333(6051): 1888-1891.
[65] Qian Y, Cosio D M O, Piatkevich K D, et al. Improved genetically encoded near-infrared fluorescent calcium ion indicators for in vivo imaging[J]. PLoS Biology, 2020, 18(11): e3000965.
[66] Qian Y, Piatkevich K D, Mc Larney B, et al. A genetically encoded near-infrared fluorescent calcium ion indicator[J]. Nature Methods, 2019, 16(2): 171-174.
[68] Shemetov A A, Monakhov M V, Zhang Q R, et al. A near-infrared genetically encoded calcium indicator for in vivo imaging[J]. Nature Biotechnology, 2021, 39(3): 368-377.
[69] Shcherbakova D M. Near-infrared and far-red genetically encoded indicators of neuronal activity[J]. Journal of Neuroscience Methods, 2021, 362: 109314.
[70] Chen T W, Wardill T J, Sun Y, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity[J]. Nature, 2013, 499(7458): 295-300.
[71] Siegel M S, Isacoff E Y. A genetically encoded optical probe of membrane voltage[J]. Neuron, 1997, 19(4): 735-741.
[72] Madhusoodanan J. Genetic light bulbs illuminate the brain[J]. Nature, 2019, 574(7778): 437-439.
[73] Knöpfel T. Genetically encoded optical indicators for the analysis of neuronal circuits[J]. Nature Reviews Neuroscience, 2012, 13(10): 687-700.
[74] Knöpfel T, Song C C. Optical voltage imaging in neurons: moving from technology development to practical tool[J]. Nature Reviews Neuroscience, 2019, 20(12): 719-727.
[75] Emiliani V, Entcheva E, Hedrich R, et al. Optogenetics for light control of biological systems[J]. Nature Reviews Methods Primers, 2022, 2(1): 55.
[76] Adam Y, Kim J J, Lou S, et al. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics[J]. Nature, 2019, 569(7756): 413-417.
[77] Fan L Z, Kheifets S, Böhm U L, et al. All-optical electrophysiology reveals the role of lateral inhibition in sensory processing in cortical layer 1[J]. Cell, 2020, 180(3): 521-535.
[78] Lin M Z, Schnitzer M J. Genetically encoded indicators of neuronal activity[J]. Nature Neuroscience, 2016, 19(9): 1142-1153.
[79] Pal A, Tian L. Imaging voltage and brain chemistry with genetically encoded sensors and modulators[J]. Current Opinion in Chemical Biology, 2020, 57: 166-176.
[80] Panzera L C, Hoppa M B. Genetically encoded voltage indicators are illuminating subcellular physiology of the axon[J]. Frontiers in Cellular Neuroscience, 2019, 13: 52.
[81] Wang W J, Kim C K, Ting A Y. Molecular tools for imaging and recording neuronal activity[J]. Nature Chemical Biology, 2019, 15(2): 101-110.
[82] Cosco E D, Arús B A, Spearman A L, et al. Bright chromenylium polymethine dyes enable fast, four-color in vivo imaging with shortwave infrared detection[J]. Journal of the American Chemical Society, 2021, 143(18): 6836-6846.
[83] Matikonda S S, Ivanic J, Gomez M, et al. Core remodeling leads to long wavelength fluoro-coumarins[J]. Chemical Science, 2020, 11(28): 7302-7307.
[84] Cornejo V H, Ofer N, Yuste R. Voltage compartmentalization in dendritic spines in vivo[J]. Science, 2022, 375(6576): 82-86.
[85] Tian H, Davis H C, Wong-Campos J D, et al. Video-based pooled screening yields improved far-red genetically encoded voltage indicators[J]. Nature Methods, 2023: 1-13.
[86] Wang M J, Da Y F, Tian Y. Fluorescent proteins and genetically encoded biosensors[J]. Chemical Society Reviews, 2023, 52(4): 1189-1214.
[87] Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics[J]. Annual Review of Neuroscience, 2011, 34: 389-412.
[88] Nagel G, Szellas T, Huhn W, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel[J]. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(24): 13940-13945.
[89] Nagel G, Ollig D, Fuhrmann M, et al. Channelrhodopsin-1: a light-gated proton channel in green algae[J]. Science, 2002, 296(5577): 2395-2398.
[90] Boyden E S, Zhang F, Bamberg E, et al. Millisecond-timescale, genetically targeted optical control of neural activity[J]. Nature Neuroscience, 2005, 8(9): 1263-1268.
[91] Petreanu L, Huber D, Sobczyk A, et al. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections[J]. Nature Neuroscience, 2007, 10(5): 663-668.
[92] Lin D Y, Boyle M P, Dollar P, et al. Functional identification of an aggression locus in the mouse hypothalamus[J]. Nature, 2011, 470(7333): 221-226.
[93] Paz J T, Davidson T J, Frechette E S, et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury[J]. Nature Neuroscience, 2013, 16(1): 64-70.
[94] Busskamp V, Duebel J, Balya D, et al. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa[J]. Science, 2010, 329(5990): 413-417.
[95] Sahel J A, Boulanger-Scemama E, Pagot C, et al. Partial recovery of visual function in a blind patient after optogenetic therapy[J]. Nature Medicine, 2021, 27(7): 1223-1229.
[96] Busskamp V, Picaud S, Sahel J A, et al. Optogenetic therapy for retinitis pigmentosa[J]. Gene Therapy, 2012, 19(2): 169-175.
[97] Wietek J, Wiegert J S, Adeishvili N, et al. Conversion of channelrhodopsin into a light-gated chloride channel[J]. Science, 2014, 344(6182): 409-412.
[98] Lin J Y, Knutsen P M, Muller A, et al. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation[J]. Nature Neuroscience, 2013, 16(10): 1499-1508.
[99] Wietek J, Rodriguez-Rozada S, Tutas J, et al. Anion-conducting channelrhodopsins with tuned spectra and modified kinetics engineered for optogenetic manipulation of behavior[J]. Scientific Reports, 2017, 7(1): 1-18.
[100] Marshel J H, Kim Y S, Machado T A, et al. Cortical layer-specific critical dynamics triggering perception[J]. Science, 2019, 365(6453): eaaw5202.
[101] Klapoetke N C, Murata Y, Kim S S, et al. Independent optical excitation of distinct neural populations[J]. Nature Methods, 2014, 11(3): 338-346.
[102] Mager T, Lopez de la Morena D, Senn V, et al. High frequency neural spiking and auditory signaling by ultrafast red-shifted optogenetics[J]. Nature Communications, 2018, 9(1): 1-14.
[103] Lehtinen K, Nokia M S, Takala H. Red light optogenetics in neuroscience[J]. Frontiers in Cellular Neuroscience, 2022, 15: 778900.
[104] Attwell D, Buchan A M, Charpak S, et al. Glial and neuronal control of brain blood flow[J]. Nature, 2010, 468(7321): 232-243.
[105] Muoio V, Persson P B, Sendeski M M. The neurovascular unit - concept review[J]. Acta Physiologica, 2014, 210(4): 790-798.
[106] Kazmi S M, Richards L M, Schrandt C J, et al. Expanding applications, accuracy, and interpretation of laser speckle contrast imaging of cerebral blood flow[J]. Journal of Cerebral Blood Flow and Metabolism, 2015, 35(7): 1076-1084.
[107] 李晨曦, 陈文亮, 蒋景英, 等. 激光散斑衬比血流成像技术研究进展[J]. 中国激光, 2018, 45(2): 0207006.
[108] Glover G H. Overview of functional magnetic resonance imaging[J]. Neurosurgery Clinics of North America, 2011, 22(2): 133-139.
[109] Kim T N, Goodwill P W, Chen Y N, et al. Line-scanning particle image velocimetry: an optical approach for quantifying a wide range of blood flow speeds in live animals[J]. PLoS One, 2012, 7(6): e38590.
[110] Meng G H, Zhong J, Zhang Q R, et al. Ultrafast two-photon fluorescence imaging of cerebral blood circulation in the mouse brain in vivo[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(23): e2117346119.
[111] Lecoq J, Parpaleix A, Roussakis E, et al. Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels[J]. Nature Medicine, 2011, 17(7): 893-898.
[112] DrexlerW, FujimotoJ G. Optical coherence tomography imaging: technology and applications[M]∥Optical coherence tomography. Cham: Springer, 2015: 1685-1735.
[113] Baran U, Wang R K. Review of optical coherence tomography based angiography in neuroscience[J]. Neurophotonics, 2016, 3(1): 010902.
[114] Wang L V, Yao J J. A practical guide to photoacoustic tomography in the life sciences[J]. Nature Methods, 2016, 13(8): 627-638.
[115] Ma Y, Shaik M A, Kim S H, et al. Wide-field optical mapping of neural activity and brain haemodynamics: considerations and novel approaches[J]. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 2016, 371(1705): 20150360.
[116] Fantini S, Frederick B, Sassaroli A. Perspective: prospects of non-invasive sensing of the human brain with diffuse optical imaging[J]. APL Photonics, 2018, 3(11): 110901.
[117] Juavinett A L, Nauhaus I, Garrett M E, et al. Automated identification of mouse visual areas with intrinsic signal imaging[J]. Nature Protocols, 2017, 12(1): 32-43.
[118] Nakamichi Y, Okubo K, Sato T, et al. Optical intrinsic signal imaging with optogenetics reveals functional cortico-cortical connectivity at the columnar level in living macaques[J]. Scientific Reports, 2019, 9(1): 6466.
[119] Chen S B, Li P C, Luo W H, et al. Time-varying spreading depression waves in rat cortex revealed by optical intrinsic signal imaging[J]. Neuroscience Letters, 2006, 396(2): 132-136.
[120] 龙晓云, 田超. 生物医学光声显微成像: 技术和应用进展[J]. 中国激光, 2020, 47(2): 0207016.
[121] Wu J L, Ji N, Tsia K K. Speed scaling in multiphoton fluorescence microscopy[J]. Nature Photonics, 2021, 15(11): 800-812.
[122] Yang W J, Yuste R. In vivo imaging of neural activity[J]. Nature Methods, 2017, 14(4): 349-359.
[123] Cramer S W, Carter R E, Aronson J D, et al. Through the looking glass: a review of cranial window technology for optical access to the brain[J]. Journal of Neuroscience Methods, 2021, 354: 109100.
[124] Huang S H, Irawati N, Chien Y F, et al. Optical volumetric brain imaging: speed, depth, and resolution enhancement[J]. Journal of Physics D: Applied Physics, 2021, 54(32): 323002.
[125] Scheele C L G J, Herrmann D, Yamashita E, et al. Multiphoton intravital microscopy of rodents[J]. Nature Reviews Methods Primers, 2022, 2: 89.
[126] 王少伟, 雷铭. 近红外二区激发多光子荧光成像[J]. 激光与光电子学进展, 2022, 59(6): 0617002.
[127] Jennings J H, Kim C K, Marshel J H, et al. Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour[J]. Nature, 2019, 565(7741): 645-649.
[128] He F, Sun Y C, Jin Y F, et al. Longitudinal neural and vascular recovery following ultraflexible neural electrode implantation in aged mice[J]. Biomaterials, 2022, 291: 121905.
[129] Skocek O, Nöbauer T, Weilguny L, et al. High-speed volumetric imaging of neuronal activity in freely moving rodents[J]. Nature Methods, 2018, 15(6): 429-432.
[130] Dussaux C, Szabo V, Chastagnier Y, et al. Fast confocal fluorescence imaging in freely behaving mice[J]. Scientific Reports, 2018, 8(1): 1-14.
[131] Fan J T, Suo J L, Wu J M, et al. Video-rate imaging of biological dynamics at centimetre scale and micrometre resolution[J]. Nature Photonics, 2019, 13(11): 809-816.
[132] Yu C H, Stirman J N, Yu Y Y, et al. Diesel2p mesoscope with dual independent scan engines for flexible capture of dynamics in distributed neural circuitry[J]. Nature Communications, 2021, 12(1): 1-8.
[133] Voleti V, Patel K B, Li W Z, et al. Real-time volumetric microscopy of in vivo dynamics and large-scale samples with SCAPE 2.0[J]. Nature Methods, 2019, 16(10): 1054-1062.
[134] Bouchard M B, Voleti V, Mendes C S, et al. Swept confocally-aligned planar excitation (SCAPE) microscopy for high-speed volumetric imaging of behaving organisms[J]. Nature Photonics, 2015, 9(2): 113-119.
[135] Keller P J, Schmidt A D, Wittbrodt J, et al. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy[J]. Science, 2008, 322(5904): 1065-1069.
[136] Stelzer E H K. Light-sheet fluorescence microscopy for quantitative biology[J]. Nature Methods, 2015, 12(1): 23-26.
[137] LevoyM, NgR, AdamsA, et al. Light field microscopy[C]∥SIGGRAPH 06: ACM SIGGRAPH 2006 Papers, July 30, 2006, Boston, Massachusetts. New York: ACM Press, 2006: 924-934.
[138] Prevedel R, Yoon Y G, Hoffmann M, et al. Simultaneous whole-animal 3D imaging of neuronal activity using light-field microscopy[J]. Nature Methods, 2014, 11(7): 727-730.
[139] Wu J M, Lu Z, Jiang D, et al. Iterative tomography with digital adaptive optics permits hour-long intravital observation of 3D subcellular dynamics at millisecond scale[J]. Cell, 2021, 184(12): 3318-3332.
[140] Lu Z, Cai Y Y, Nie Y X, et al. A practical guide to scanning light-field microscopy with digital adaptive optics[J]. Nature Protocols, 2022, 17(9): 1953-1979.
[141] Ghosh K K, Burns L D, Cocker E D, et al. Miniaturized integration of a fluorescence microscope[J]. Nature Methods, 2011, 8(10): 871-878.
[142] Zong W J, Wu R L, Li M L, et al. Fast high-resolution miniature two-photon microscopy for brain imaging in freely-behaving mice[J]. Nature Methods, 2017, 14(7): 713-719.
[143] Trevathan J K, Asp A J, Nicolai E N, et al. Calcium imaging in freely-moving mice during electrical stimulation of deep brain structures[J]. Journal of Neural Engineering, 2021, 18(2): 026008.
[144] Xue Y J, Davison I G, Boas D A, et al. Single-shot 3D wide-field fluorescence imaging with a Computational Miniature Mesoscope[J]. Science Advances, 2020, 6(43): eabb7508.
[145] Zong W J, Obenhaus H A, Skytøen E R, et al. Large-scale two-photon calcium imaging in freely moving mice[J]. Cell, 2022, 185(7): 1240-1256.
[146] Accanto N, Blot F G C, Lorca-Cámara A, et al. A flexible two-photon fiberscope for fast activity imaging and precise optogenetic photostimulation of neurons in freely moving mice[J]. Neuron, 2023, 111(2): 176-189.
[147] Denk W, Strickler J H, Webb W W. Two-photon laser scanning fluorescence microscopy[J]. Science, 1990, 248(4951): 73-76.
[148] Helmchen F, Fee M S, Tank D W, et al. A miniature head-mounted two-photon microscope. high-resolution brain imaging in freely moving animals[J]. Neuron, 2001, 31(6): 903-912.
[149] Zong W J, Wu R L, Chen S Y, et al. Miniature two-photon microscopy for enlarged field-of-view, multi-plane and long-term brain imaging[J]. Nature Methods, 2021, 18(1): 46-49.
[150] Klioutchnikov A, Wallace D J, Sawinski J, et al. A three-photon head-mounted microscope for imaging all layers of visual cortex in freely moving mice[J]. Nature Methods, 2022: 1-7.
[151] Guan H H, Li D W, Park H C, et al. Deep-learning two-photon fiberscopy for video-rate brain imaging in freely-behaving mice[J]. Nature Communications, 2022, 13(1): 1-9.
[152] Sun J W, Wu J C, Wu S, et al. Quantitative phase imaging through an ultra-thin lensless fiber endoscope[J]. Light: Science & Applications, 2022, 11(1): 1-10.
[154] Nöbauer T, Skocek O, Pernía-Andrade A J, et al. Video rate volumetric Ca2+ imaging across cortex using seeded iterative demixing (SID) microscopy[J]. Nature Methods, 2017, 14(8): 811-818.
[155] Xue Y J, Yang Q W, Hu G R, et al. Deep-learning-augmented computational miniature mesoscope[J]. Optica, 2022, 9(9): 1009-1021.
[156] Adams J K, Boominathan V, Avants B W, et al. Single-frame 3D fluorescence microscopy with ultraminiature lensless FlatScope[J]. Science Advances, 2017, 3(12): e1701548.
[157] Adams J K, Yan D, Wu J M, et al. In vivo lensless microscopy via a phase mask generating diffraction patterns with high-contrast contours[J]. Nature Biomedical Engineering, 2022, 6(5): 617-628.
[158] Boominathan V, Robinson J T, Waller L, et al. Recent advances in lensless imaging[J]. Optica, 2022, 9(1): 1-16.
[159] Hodgkin A L, Huxley A F. Action potentials recorded from inside a nerve fibre[J]. Nature, 1939, 144(3651): 710-711.
[160] Hodgkin A L, Huxley A F. A quantitative description of membrane current and its application to conduction and excitation in nerve[J]. The Journal of Physiology, 1952, 117(4): 500-544.
[161] Gesteland R C, Howland B, Lettvin J Y, et al. Comments on microelectrodes[J]. Proceedings of the The Institute of Radio Engineers, 1959, 47(11): 1856-1862.
[162] EjserholmF. Development of a polymer based neural probe-How to record intracortical neural activity while minimizing the tissue response[M]. Lund University, 2016.
[163] Weltman A, Yoo J, Meng E. Flexible, penetrating brain probes enabled by advances in polymer microfabrication[J]. Micromachines, 2016, 7(10): 180.
[164] Wise K D, Angell J B, Starr A. An integrated-circuit approach to extracellular microelectrodes[J]. IEEE Transactions on Bio-Medical Engineering, 1970, 17(3): 238-247.
[165] Maynard E M, Nordhausen C T, Normann R A. The Utah intracortical electrode array: a recording structure for potential brain-computer interfaces[J]. Electroencephalography and Clinical Neurophysiology, 1997, 102(3): 228-239.
[166] Jun J J, Steinmetz N A, Siegle J H, et al. Fully integrated silicon probes for high-density recording of neural activity[J]. Nature, 2017, 551(7679): 232-236.
[167] Khodagholy D, Gelinas J N, Thesen T, et al. NeuroGrid: recording action potentials from the surface of the brain[J]. Nature Neuroscience, 2015, 18(2): 310-315.
[168] Minev I R, Musienko P, Hirsch A, et al. Electronic dura mater for long-term multimodal neural interfaces[J]. Science, 2015, 347(6218): 159-163.
[169] Liu J, Fu T M, Cheng Z G, et al. Syringe-injectable electronics[J]. Nature Nanotechnology, 2015, 10(7): 629-636.
[170] Yang X, Zhou T, Zwang T J, et al. Bioinspired neuron-like electronics[J]. Nature Materials, 2019, 18(5): 510-517.
[171] Luan L, Wei X L, Zhao Z T, et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration[J]. Science Advances, 2017, 3(2): e1601966.
[172] Guan S, Wang J, Gu X, et al. Elastocapillary self-assembled neurotassels for stable neural activity recordings[J]. Science Advances, 2019, 5(3): eaav2842.
[173] Musk E, Neuralink. An integrated brain-machine interface platform with thousands of channels[J]. Journal of Medical Internet Research, 2019, 21(10): e16194.
[174] Zhao Z T, Zhu H L, Li X, et al. Ultraflexible electrode arrays for months-long high-density electrophysiological mapping of thousands of neurons in rodents[J]. Nature Biomedical Engineering, 2022: 1-13.
[175] Buzsáki G, Anastassiou C A, Koch C. The origin of extracellular fields and currents: EEG, ECoG, LFP and spikes[J]. Nature Reviews Neuroscience, 2012, 13(6): 407-420.
[176] Galvani L. De viribus electricitatis in motu musculari commentarius[J]. Commentarii de Bononiensi Scientiarum et Artium Instituto Atque Academia, 1791, 7: 363-418.
[177] Woods G A, Rommelfanger N J, Hong G S. Bioinspired materials for in vivo bioelectronic neural interfaces[J]. Matter, 2020, 3(4): 1087-1113.
[178] Gray C M, Maldonado P E, Wilson M, et al. Tetrodes markedly improve the reliability and yield of multiple single-unit isolation from multi-unit recordings in cat striate cortex[J]. Journal of Neuroscience Methods, 1995, 63(1/2): 43-54.
[179] Abidian M R, Martin D C. Multifunctional nanobiomaterials for neural interfaces[J]. Advanced Functional Materials, 2009, 19(4): 573-585.
[180] Steinmetz N A, Aydin C, Lebedeva A, et al. Neuropixels 2.0: a miniaturized high-density probe for stable, long-term brain recordings[J]. Science, 2021, 372(6539): eabf4588.
[181] Chiang C H, Won S M, Orsborn A L, et al. Development of a neural interface for high-definition, long-term recording in rodents and nonhuman primates[J]. Science Translational Medicine, 2020, 12(538): eaay4682.
[182] Xie C, Liu J, Fu T M, et al. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes[J]. Nature Materials, 2015, 14(12): 1286-1292.
[183] Spix T A, Nanivadekar S, Toong N, et al. Population-specific neuromodulation prolongs therapeutic benefits of deep brain stimulation[J]. Science, 2021, 374(6564): 201-206.
[184] Abdelfattah A, Ahuja S, Akkin T, et al. Neurophotonic tools for microscopic measurements and manipulation: status report[J]. Neurophotonics, 2022, 9(S1): 013001.
[185] Guo Q C, Zhou J F, Feng Q R, et al. Multi-channel fiber photometry for population neuronal activity recording[J]. Biomedical Optics Express, 2015, 6(10): 3919-3931.
[186] Aravanis A M, Wang L P, Zhang F, et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology[J]. Journal of Neural Engineering, 2007, 4(3): S143-S156.
[187] Li Y M, Wang Y, Chen H, et al. Development of implantable optrode devices[J]. Acta Physico-Chimica Sinica, 2020, 36(12): 1912054.
[188] Pashaie R, Falk R. Single optical fiber probe for fluorescence detection and optogenetic stimulation[J]. IEEE Transactions on Bio-Medical Engineering, 2013, 60(2): 268-280.
[189] Dubois A, Chiang C C, Smekens F, et al. Optical and thermal simulations for the design of optodes for minimally invasive optogenetics stimulation or photomodulation of deep and large cortical areas in non-human primate brain[J]. Journal of Neural Engineering, 2018, 15(6): 065004.
[190] Szabo V, Ventalon C, De Sars V, et al. Spatially selective holographic photoactivation and functional fluorescence imaging in freely behaving mice with a fiberscope[J]. Neuron, 2014, 84(6): 1157-1169.
[191] Kim C K, Adhikari A, Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience[J]. Nature Reviews Neuroscience, 2017, 18(4): 222-235.
[192] Schlegel F, Sych Y, Schroeter A, et al. Fiber-optic implant for simultaneous fluorescence-based calcium recordings and BOLD fMRI in mice[J]. Nature Protocols, 2018, 13(5): 840-855.
[193] Tsakas A, Tselios C, Ampeliotis D, et al. Review of optical fiber technologies for optogenetics[J]. Results in Optics, 2021, 5: 100168.
[194] Fu R X, Luo W H, Nazempour R, et al. Implantable and biodegradable poly(l-lactic acid) fibers for optical neural interfaces[J]. Advanced Optical Materials, 2018, 6(3): 1700941.
[195] Pisanello F, Sileo L, Oldenburg I, et al. Multipoint-emitting optical fibers for spatially addressable in vivo optogenetics[J]. Neuron, 2014, 82(6): 1245-1254.
[196] Pisanello F, Mandelbaum G, Pisanello M, et al. Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber[J]. Nature Neuroscience, 2017, 20(8): 1180-1188.
[197] Pisano F, Pisanello M, Lee S J, et al. Depth-resolved fiber photometry with a single tapered optical fiber implant[J]. Nature Methods, 2019, 16(11): 1185-1192.
[198] Moreaux L C, Yatsenko D, Sacher W D, et al. Integrated neurophotonics: toward dense volumetric interrogation of brain circuit activity-at depth and in real time[J]. Neuron, 2020, 108(1): 66-92.
[199] Parbrook P J, Corbett B, Han J, et al. Micro-light emitting diode: from chips to applications[J]. Laser & Photonics Reviews, 2021, 15(5): 2000133.
[200] Wu F, Stark E, Ku P C, et al. Monolithically integrated μLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals[J]. Neuron, 2015, 88(6): 1136-1148.
[201] Kim T I, McCall J G, Jung Y H, et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics[J]. Science, 2013, 340(6129): 211-216.
[202] Son Y, Lee H J, Kim J, et al. In vivo optical modulation of neural signals using monolithically integrated two-dimensional neural probe arrays[J]. Scientific Reports, 2015, 5(1): 1-11.
[203] Canales A, Jia X T, Froriep U P, et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo[J]. Nature Biotechnology, 2015, 33(3): 277-284.
[204] Spagnolo B, Balena A, Peixoto R T, et al. Tapered fibertrodes for optoelectrical neural interfacing in small brain volumes with reduced artefacts[J]. Nature Materials, 2022, 21(7): 826-835.
[205] Lu L Y, Gutruf P, Xia L, et al. Wireless optoelectronic photometers for monitoring neuronal dynamics in the deep brain[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(7): E1374-E1383.
[206] Yang Y Y, Wu M Z, Vázquez-Guardado A, et al. Wireless multilateral devices for optogenetic studies of individual and social behaviors[J]. Nature Neuroscience, 2021, 24(7): 1035-1045.
[207] Huang Y X, Cui Y T, Deng H J, et al. Bioresorbable thin-film silicon diodes for the optoelectronic excitation and inhibition of neural activities[J]. Nature Biomedical Engineering, 2022: 1-13.
[208] Li L Z, Lu L H, Ren Y Q, et al. Colocalized, bidirectional optogenetic modulations in freely behaving mice with a wireless dual-color optoelectronic probe[J]. Nature Communications, 2022, 13(1): 1-14.
[209] Cai X, Li L Z, Liu W H, et al. A dual-channel optogenetic stimulator selectively modulates distinct defensive behaviors[J]. iScience, 2022, 25(1): 103681.
[210] Qazi R, Kim C Y, Byun S H, et al. Microscale inorganic LED based wireless neural systems for chronic in vivo optogenetics[J]. Frontiers in Neuroscience, 2018, 12: 764.
[211] Park S, Guo Y Y, Jia X T, et al. One-step optogenetics with multifunctional flexible polymer fibers[J]. Nature Neuroscience, 2017, 20(4): 612-619.
[212] Lee W, Kim D, Matsuhisa N, et al. Transparent, conformable, active multielectrode array using organic electrochemical transistors[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(40): 10554-10559.
[213] Kayser L V, Lipomi D J. Stretchable conductive polymers and composites based on PEDOT and PEDOT: PSS[J]. Advanced Materials, 2019, 31(10): e1806133.
[214] Michelson N J, Eles J R, Vazquez A L, et al. Calcium activation of cortical neurons by continuous electrical stimulation: frequency dependence, temporal fidelity, and activation density[J]. Journal of Neuroscience Research, 2019, 97(5): 620-638.
[215] Wei X L, Luan L, Zhao Z T, et al. Nanofabricated ultraflexible electrode arrays for high-density intracortical recording[J]. Advanced Science, 2018, 5(6): 1700625.
[216] Liu X, Ren C, Lu Y C, et al. Multimodal neural recordings with Neuro-FITM uncover diverse patterns of cortical-hippocampal interactions[J]. Nature Neuroscience, 2021, 24(6): 886-896.
[217] Wu X T, Yang X Y, Song L L, et al. A modified miniscope system for simultaneous electrophysiology and calcium imaging in vivo[J]. Frontiers in Integrative Neuroscience, 2021, 15: 682019.
[218] Ghanbari L, Carter R E, Rynes M L, et al. Cortex-wide neural interfacing via transparent polymer skulls[J]. Nature Communications, 2019, 10(1): 1-13.
[219] Kim T H, Zhang Y P, Lecoq J, et al. Long-term optical access to an estimated one million neurons in the live mouse cortex[J]. Cell Reports, 2016, 17(12): 3385-3394.
[220] Kozai T D Y, Eles J R, Vazquez A L, et al. Two-photon imaging of chronically implanted neural electrodes: Sealing methods and new insights[J]. Journal of Neuroscience Methods, 2016, 258: 46-55.
[222] Poskanzer K E, Yuste R. Astrocytes regulate cortical state switching in vivo[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(19): E2675-E2684.
[223] Dunn A K, Bolay H, Moskowitz M A, et al. Dynamic imaging of cerebral blood flow using laser speckle[J]. Journal of Cerebral Blood Flow & Metabolism, 2001, 21(3): 195-201.
[224] McCullough C M, Ramirez-Gordillo D, Hall M, et al. GRINtrode: a neural implant for simultaneous two-photon imaging and extracellular electrophysiology in freely moving animals[J]. Neurophotonics, 2022, 9(4): 045009.
[225] Park D W, Brodnick S K, Ness J P, et al. Fabrication and utility of a transparent graphene neural electrode array for electrophysiology, in vivo imaging, and optogenetics[J]. Nature Protocols, 2016, 11(11): 2201-2222.
[226] Thunemann M, Lu Y C, Liu X, et al. Deep 2-photon imaging and artifact-free optogenetics through transparent graphene microelectrode arrays[J]. Nature Communications, 2018, 9(1): 1-12.
[227] Driscoll N, Rosch R E, Murphy B B, et al. Multimodal in vivo recording using transparent graphene microelectrodes illuminates spatiotemporal seizure dynamics at the microscale[J]. Communications Biology, 2021, 4(1): 1-14.
[228] Kuzum D, Takano H, Shim E, et al. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging[J]. Nature Communications, 2014, 5(1): 1-10.
[229] Park D W, Schendel A A, Mikael S, et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications[J]. Nature Communications, 2014, 5(1): 1-11.
[230] Jang H, Park Y J, Chen X, et al. Graphene-based flexible and stretchable electronics[J]. Advanced Materials, 2016, 28(22): 4184-4202.
[231] Pashaie R, Anikeeva P, Lee J H, et al. Optogenetic brain interfaces[J]. IEEE Reviews in Biomedical Engineering, 2014, 7: 3-30.
[232] Richner T J, Baumgartner R, Brodnick S K, et al. Patterned optogenetic modulation of neurovascular and metabolic signals[J]. Journal of Cerebral Blood Flow and Metabolism, 2015, 35(1): 140-147.
[233] Lee J, Ozden I, Song Y K, et al. Transparent intracortical microprobe array for simultaneous spatiotemporal optical stimulation and multichannel electrical recording[J]. Nature Methods, 2015, 12(12): 1157-1162.
[234] Wang Y, Zhu C X, Pfattner R, et al. A highly stretchable, transparent, and conductive polymer[J]. Science Advances, 2017, 3(3): e1602076.
[235] Lu H Y, Lorenc E S, Zhu H L, et al. Multi-scale neural decoding and analysis[J]. Journal of Neural Engineering, 2021, 18(4): 045013.
[236] Petersen P C, Siegle J H, Steinmetz N A, et al. CellExplorer: a framework for visualizing and characterizing single neurons[J]. Neuron, 2021, 109(22): 3594-3608.
[237] Dai X C, Zhou W, Gao T, et al. Three-dimensional mapping and regulation of action potential propagation in nanoelectronics-innervated tissues[J]. Nature Nanotechnology, 2016, 11(9): 776-782.
[238] Benisty H, Song A, Mishne G, et al. Review of data processing of functional optical microscopy for neuroscience[J]. Neurophotonics, 2022, 9(4): 041402.
[239] Tahir W, Kura S, Zhu J B, et al. Anatomical modeling of brain vasculature in two-photon microscopy by generalizable deep learning[J]. BME Frontiers, 2020, 2020: 8620932.
[242] Bao Y J, Soltanian-Zadeh S, Farsiu S, et al. Segmentation of neurons from fluorescence calcium recordings beyond real-time[J]. Nature Machine Intelligence, 2021, 3(7): 590-600.
[243] Lecoq J, Oliver M, Siegle J H, et al. Removing independent noise in systems neuroscience data using DeepInterpolation[J]. Nature Methods, 2021, 18(11): 1401-1408.
[244] Rupprecht P, Carta S, Hoffmann A, et al. A database and deep learning toolbox for noise-optimized, generalized spike inference from calcium imaging[J]. Nature Neuroscience, 2021, 24(9): 1324-1337.
[245] Wang Z Q, Zhu L X, Zhang H, et al. Real-time volumetric reconstruction of biological dynamics with light-field microscopy and deep learning[J]. Nature Methods, 2021, 18(5): 551-556.
[246] Wagner N, Beuttenmueller F, Norlin N, et al. Deep learning-enhanced light-field imaging with continuous validation[J]. Nature Methods, 2021, 18(5): 557-563.
[247] Qiao C, Li D, Guo Y T, et al. Evaluation and development of deep neural networks for image super-resolution in optical microscopy[J]. Nature Methods, 2021, 18(2): 194-202.
[248] Chung J E, Magland J F, Barnett A H, et al. A fully automated approach to spike sorting[J]. Neuron, 2017, 95(6): 1381-1394.
[249] Buccino A P, Hurwitz C L, Garcia S, et al. SpikeInterface, a unified framework for spike sorting[J]. eLife, 2020, 9: e61834.
[250] Luan L, Robinson J T, Aazhang B, et al. Recent advances in electrical neural interface engineering: minimal invasiveness, longevity, and scalability[J]. Neuron, 2020, 108(2): 302-321.
[251] Juergens E, Guettler A, Eckhorn R. Visual stimulation elicits locked and induced gamma oscillations in monkey intracortical- and EEG-potentials, but not in human EEG[J]. Experimental Brain Research, 1999, 129(2): 247-259.
[252] Mathis A, Mamidanna P, Cury K M, et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning[J]. Nature Neuroscience, 2018, 21(9): 1281-1289.
[253] Nath T, Mathis A, Chen A C, et al. Using DeepLabCut for 3D markerless pose estimation across species and behaviors[J]. Nature Protocols, 2019, 14(7): 2152-2176.
[254] Mathis M W, Mathis A. Deep learning tools for the measurement of animal behavior in neuroscience[J]. Current Opinion in Neurobiology, 2020, 60: 1-11.
[255] Pereira T D, Shaevitz J W, Murthy M. Quantifying behavior to understand the brain[J]. Nature Neuroscience, 2020, 23(12): 1537-1549.
[256] Kleinfeld D, Luan L, Mitra P P, et al. Can one concurrently record electrical spikes from every neuron in a mammalian brain?[J]. Neuron, 2019, 103(6): 1005-1015.
[257] Abbott J, Ye T Y, Krenek K, et al. A nanoelectrode array for obtaining intracellular recordings from thousands of connected neurons[J]. Nature Biomedical Engineering, 2020, 4(2): 232-241.
[258] Schoonover C E, Ohashi S N, Axel R, et al. Representational drift in primary olfactory cortex[J]. Nature, 2021, 594(7864): 541-546.
[259] Paulk A C, Kfir Y, Khanna A R, et al. Large-scale neural recordings with single neuron resolution using Neuropixels probes in human cortex[J]. Nature Neuroscience, 2022, 25(2): 252-263.
[260] Gardner R J, Hermansen E, Pachitariu M, et al. Toroidal topology of population activity in grid cells[J]. Nature, 2022, 602(7895): 123-128.
[261] Vöröslakos M, Kim K, Slager N, et al. HectoSTAR μLED optoelectrodes for large-scale, high-precision in vivo opto-electrophysiology[J]. Advanced Science, 2022, 9(18): e2105414.
[262] Chung J E, Sellers K K, Leonard M K, et al. High-density single-unit human cortical recordings using the Neuropixels probe[J]. Neuron, 2022, 110(15): 2409-2421.
[263] Grewe B F, Langer D, Kasper H, et al. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision[J]. Nature Methods, 2010, 7(5): 399-405.
[264] Ducros M, Houssen Y G, Bradley J, et al. Encoded multisite two-photon microscopy[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(32): 13138-13143.
[265] Yang W J, Miller J E, Carrillo-Reid L, et al. Simultaneous multi-plane imaging of neural circuits[J]. Neuron, 2016, 89(2): 269-284.
[267] Stirman J N, Smith I T, Kudenov M W, et al. Wide field-of-view, multi-region two-photon imaging of neuronal activity in the mammalian brain[J]. Nature Biotechnology, 2016, 34: 857-862.
[268] Nadella K M N S, Roš H, Baragli C, et al. Random-access scanning microscopy for 3D imaging in awake behaving animals[J]. Nature Methods, 2016, 13(12): 1001-1004.
[269] Prevedel R, Verhoef A J, Pernía-Andrade A J, et al. Fast volumetric calcium imaging across multiple cortical layers using sculpted light[J]. Nature Methods, 2016, 13(12): 1021-1028.
[270] Lu R, Sun W, Liang Y, et al. Video-rate volumetric functional imaging of the brain at synaptic resolution[J]. Nature Neuroscience, 2017, 20: 620-628.
[271] Kazemipour A, Novak O, Flickinger D, et al. Kilohertz frame-rate two-photon tomography[J]. Nature Methods, 2019, 16(8): 778-786.
[272] Demas J, Manley J, Tejera F, et al. High-speed, cortex-wide volumetric recording of neuroactivity at cellular resolution using light beads microscopy[J]. Nature Methods, 2021, 18(9): 1103-1111.
[273] Durand S, Heller G R, Ramirez T K, et al. Acute head-fixed recordings in awake mice with multiple Neuropixels probes[J]. Nature Protocols, 2023, 18(2): 424-457.
[274] Bansal A, Shikha S, Zhang Y. Towards translational optogenetics[J]. Nature Biomedical Engineering, 2022: 1-21.
[275] Okano H. Current status of and perspectives on the application of marmosets in neurobiology[J]. Annual Review of Neuroscience, 2021, 44: 27-48.
[276] Hussey G S, Dziki J L, Badylak S F. Extracellular matrix-based materials for regenerative medicine[J]. Nature Reviews Materials, 2018, 3(7): 159-173.
[277] Kang S K, Murphy R K J, Hwang S W, et al. Bioresorbable silicon electronic sensors for the brain[J]. Nature, 2016, 530(7588): 71-76.
[278] Zaaimi B, Turnbull M, Hazra A, et al. Closed-loop optogenetic control of the dynamics of neural activity in non-human Primates[J]. Nature Biomedical Engineering, 2022: 1-17.
[279] Piech D K, Johnson B C, Shen K, et al. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication[J]. Nature Biomedical Engineering, 2020, 4(2): 207-222.
[280] Won S M, Cai L, Gutruf P, et al. Wireless and battery-free technologies for neuroengineering[J]. Nature Biomedical Engineering, 2021: 1-19.
[281] Marx V. Neuroscientists go wireless[J]. Nature Methods, 2021, 18(10): 1150-1154.
[282] Collins F S, Tabak L A. Policy: NIH plans to enhance reproducibility[J]. Nature, 2014, 505(7485): 612-613.
[283] Li X Y, Zhang G X, Wu J M, et al. Reinforcing neuron extraction and spike inference in calcium imaging using deep self-supervised denoising[J]. Nature Methods, 2021, 18(11): 1395-1400.
[284] Li X Y, Li Y X, Zhou Y L, et al. Real-time denoising enables high-sensitivity fluorescence time-lapse imaging beyond the shot-noise limit[J]. Nature Biotechnology, 2023, 41(2): 282-292.
[285] Sun F M, Zeng J Z, Jing M, et al. A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice[J]. Cell, 2018, 174(2): 481-496.
[286] Jing M, Zhang P, Wang G F, et al. A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies[J]. Nature Biotechnology, 2018, 36(8): 726-737.
[287] Feng J S, Zhang C M, Lischinsky J E, et al. A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine[J]. Neuron, 2019, 102(4): 745-761.
[288] Sun F M, Zhou J H, Dai B, et al. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo[J]. Nature Methods, 2020, 17(11): 1156-1166.
[289] Wan J X, Peng W L, Li X L, et al. A genetically encoded sensor for measuring serotonin dynamics[J]. Nature Neuroscience, 2021, 24(5): 746-752.
[290] Dong A, He K K, Dudok B, et al. A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo[J]. Nature Biotechnology, 2022, 40(5): 787-798.
[291] Ngai J. BRAIN 2.0: transforming neuroscience[J]. Cell, 2022, 185(1): 4-8.
Article Outline
徐明亮, 李芳媛, 刘岳圻, 张瑾慧, 师亚洲, 何飞. 植入式多模态神经接口前沿进展[J]. 中国激光, 2023, 50(15): 1507301. Mingliang Xu, Fangyuan Li, Yueqi Liu, Jinhui Zhang, Yazhou Shi, Fei He. Frontiers of Implantable Multimodal Neural Interfaces[J]. Chinese Journal of Lasers, 2023, 50(15): 1507301.