NiN4/Cr修饰的石墨烯电化学固氮电极
Ammonia is an important chemical material that is widely used in industrial and agricultural production[1⇓⇓⇓-5]. Currently, ammonia is mainly produced via the Haber- Bosch process in industry by using nitrogen and hydrogen as raw materials[6⇓⇓-9]. In order to break the inherent inert N≡N triple bond, the Haber-Bosch process using Fe and Ru metal-based catalysts with promoters under the harsh reaction environment, leading to the heavy energy consumption and the massive CO2 emission[10⇓⇓-13]. Naturally, ammonia synthesis via nitrogen reduction reaction under mild conditions becomes an economic and eco-friendly way, which is inspired by the soybean rhizobium and bacteria nitrogenase[14]. Based on this strategy, exploring efficient catalysts that can effectively facilitate electrochemical nitrogen reduction reaction (NRR) is desirable, however, it remains a big challenge.
Graphene has shown attractive catalytic activity due to its extraordinary electronic, thermal and mechanical properties[15]. Considering its electron neutrality, the common strategy to activate the inert graphene is the direct introduction of the TM/N heteroatoms as the active sites. For example, Choi et al.[4] have reported that several single atom catalysts (SACs) including TiN4 and VN4 embedded graphene exhibits better activity in comparison with Ru (0001) stepped surface. Furthermore, Yang et al.[16] have reported that among varied TMN4 embedded graphene (TM = Fe, Co, Mo, W, Ru, Rh), the MoN4 site exhibits outstanding catalytic activity for ammonia synthesis with small reaction energy barrier of 0.67 eV. Analogously, Riyaz et al.[17] have demonstrated that the best activity is offered by CrN4 site among different TMN4 (TM=Cr, Mn, Fe, Mo, Ru) embedded graphene.
Compared with single-metal atom catalysts, double atom catalysts (DACs) with synergetic interatomic interactions and flexible active sites, can maximize the potentials of catalysts, which makes the optimization of activity and selectivity feasible. They have emerged as more beneficial catalysts for electrochemical reactions. For instance, Sun et al.[18] have revealed that VFe-N-C shows the best catalytic activity for electrochemical NRR with limiting potential of -0.36 V. Analogously, Zheng et al.[19] have reported that only Fe/Mn-N-C catalyst is identified to be a promising candidate for NRR. The fascinating activity modification motivates our interest on the NRR reactivity catalyzed by DACs. Interestingly, Zhou et al.[20] have successfully introduced the secondary Rh atom into the FeN4 pre-embedded graphene experimentally and the obtained FeN4/Rh sample delivers the superior electrocatalytic activity. Therefore, we are interested in the N2-to-NH3 performance of TM1N4/TM2 combination induced by the introduction of the second TM heteroatom into the TMN4 pre-doping graphene. However, it has not been explored yet.
In this study, the NRR performance of the TM1N4/TM2 embedded graphene is systematically investigated by density function theory calculations. According to our results, the NiN4/Cr combination is a potential electrocatalyst for the N2-to-NH3 conversion. Besides, the Mulliken charge analysis identifies the electron transfer between the functional graphene and the NRR intermediates. The presented results provide a theoretical guide for the catalyst synthesis experimentally.
1 Computational method
All calculations are performed within the density functional theory (DFT) framework as implemented in DMol3 code[21-22]. The generalized gradient approximation (GGA) with the Perdew-Burke-Ernzerhof (PBE) functional is employed to describe exchange-correlation interactions[23]. The DFT Semi-core Pseudopotential (DSPP) core treat method is implemented for relativistic effects, which replaces core electrons by a single effective potential and introduces some degree of relativistic corrections into the core[24]. The double numerical atomic orbital augmented by a polarization function (DNP) is chosen as the basis set[21]. A smearing of 0.005 Ha (1 Ha=27.21 eV) to the orbital occupation is applied to achieve accurate electronic convergence. In the geometry structural optimization, the convergence tolerances of energy, maximum force and displacement are 1.0×10-5 Ha, 0.02 Ha/nm and 0.0005 nm, respectively. The spin- unrestricted method is used for all calculations. A conductor-like screening model (COSMO) was used to simulate a H2O solvent environment for all calculations[25]. COSMO is a continuum model in which the solute molecule forms a cavity within the dielectric continuum. The DMol3/COSMO method has been generalized to periodic boundary cases. The dielectric constant is set as 78.54 for H2O. During the geometrical optimization, the systems are free to relax.
The 4×4 supercell is an appropriate choice in the consideration of avoiding the interaction among images and reducing the cumbersome calculation[26⇓⇓-29]. The 2 nm-thick vacuum is added to avoid the artificial interactions between the catalyst and its images in the Z direction. The adsorption energies (Eads) of NRR intermediates are calculated by
where Esystem, Ecatalyst and Em represent the total energy of the adsorption system, the catalyst and the adsorbates, respectively.
Six proton-electron transfer steps were involved in the electrochemical NH3 synthesis from N2, i.e., N2+6H++ 6e-→2NH3. Gibbs free energy change (ΔG) of each elementary step was computed by computational hydrogen electrode model (CHE) raised by Nørskov et al., which the chemical potential of (H++e-) pairs equaled to one-half of H2 at standard condition[30-31]. The ΔG was determined by
where ΔE is the reaction energy analyzed directly from the DFT computations, ΔZPE and ΔS are the zero point- energy and the entropy difference at room temperature (T=298.15 K), respectively. The zero-point energies and entropies of the NRR intermediates are calculated from the vibrational frequencies according to standard methods. Following the suggestion of Wilcox, et al.[32], the substrates are fully constrained for the frequency calculation. The term of ΔGU=-eU corresponds to the free energy contribution caused by the variation of electrode potential. ΔGpH is the pH correction of the free energy, which could be expressed as ΔGpH=2.303×kBT×pH, where kB is the Boltzmann constant and pH is set to zero. ΔG<0 corresponds to an exothermic adsorption process vice versa. Furthermore, the onset potential was defined as the applied potential (U) required such that every step in the specified mechanism is exergonic.
2 Results and discussion
The atomic structure is illustrated in Fig.1(a). TM1 denotes the TM atom in TMN4 moiety and TM2 is the secondary dopant wherein TM1 considers Mn, Fe, Co and Ni elements according to the experimental accessibility and TM2 screens 3d/4d/5d TM elements[20,33-34]. For simplicity, the TM1N4/TM2 embedded graphene is referred as TM1N4/TM2. Fig. 1 (b) provides the screening criterion. In order to reduce the cumbersome calculations, the competition between NRR and the side hydrogen evolution reaction (HER) is firstly considered via comparing the strengths of the adsorption energies Eads. Herein, Eads(N2) is greater than Eads(H), indicating the favorable nitrogen adsorption. The preferential N2 adsorption could suppress the adverse HER and boost the electrochemical NH3 synthesis[8]. Subsequently, in order to ensure the activation of N2 molecule, the Eads(N2) should be stronger than -0.6 eV[35]. Herein, the three-coordination TM2 site is identified as the active site. The strong adsorption ability originates from the donation-back-donation mechanism that accepting the lone-pair electrons of N2 with the empty orbitals of TM site and thereby donating the occupied orbital electrons into the antibonding orbitals of N2. The data of Eads(N2) and Eads(H) are listed in Table S1- S4 in the supporting materials for clear reference. According to the mentioned consideration, there are total 55 combinations identified.
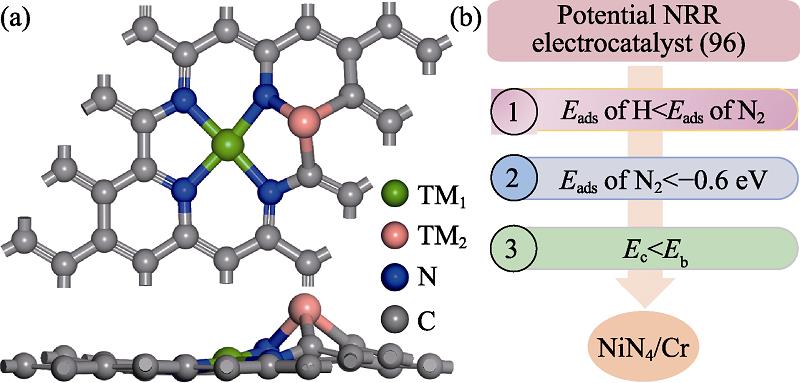
图 1.
Fig. 1. (a) Atomic structure of TM1N4/TM2 and (b) screening criterion for TM1N4/TM2 combination
According to literature data, the potential determining step (PDS) of NRR is usually located at the first protonation process, the formation of NNH[35-36]. For a quick screening, the free energy of the NNH formation (∆GN2-NNH) is evaluated in priority. The data are presented in Fig.2 where the ∆GN2-NNH value of the commercial Ru is added as reference since it is the optimal pure metal catalyst for the industrial process[37]. Therein, the most exergonic step in the reduction to form ammonia on Ru(0001) is the addition of the first hydrogen atom to form NNH and the step is 1.08 eV uphill in free energy[37].
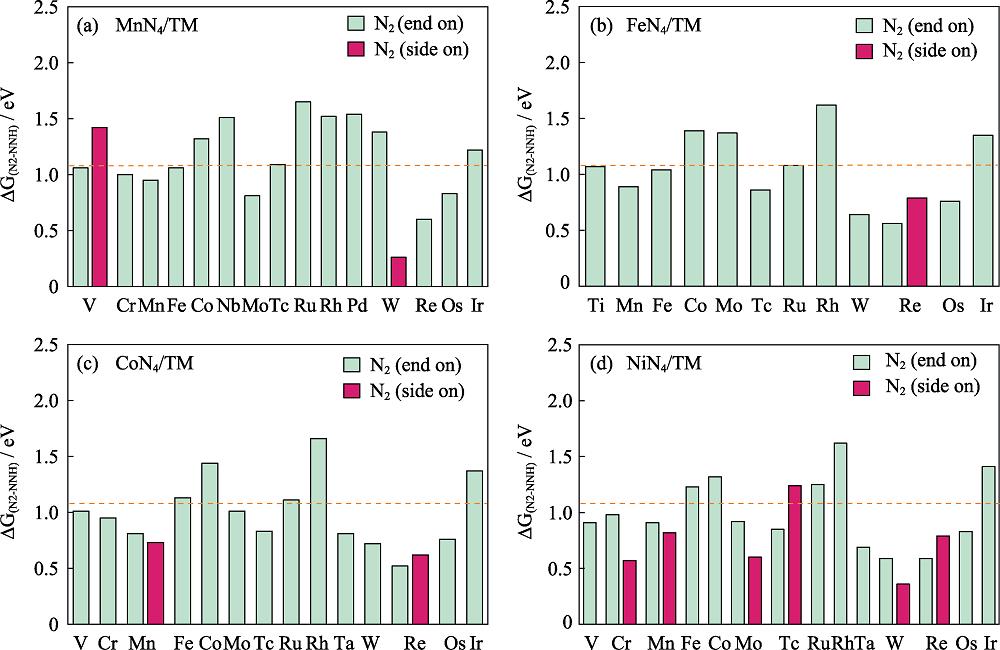
图 2.
Fig. 2. Gibbs free energy difference between N2 and NNH with the orange line at 1.08 eV Colorful figures are available on website
According to the results, we focus our attention on the systems with relatively low ∆GN2-NNH, including MnN4/W, MnN4/Re, FeN4/W, FeN4/Re, CoN4/Re, NiN4/Cr, NiN4/Mo, NiN4/Ta, NiN4/W and NiN4/Re. Noteworthy, it is necessary to test the stability of materials against the agglomeration of dispersible metal atoms. The thermodynamic stability of atomic distribution is evaluated via the binding energy Eb[38]. Fig. S1 in the supporting materials reveals that Eb of NiN4/Cr, NiN4/Mo and NiN4/Ta exceed the corresponding cohesive energy Ec, indicating the good resistance against clustering. According to the mentioned discussion, the potential electrode materials are identified as NiN4/Cr, NiN4/Mo and NiN4/Ta. Therefore, we perform the complete free energy profiles of the NRR progress focused on the three candidates in the following discussion.
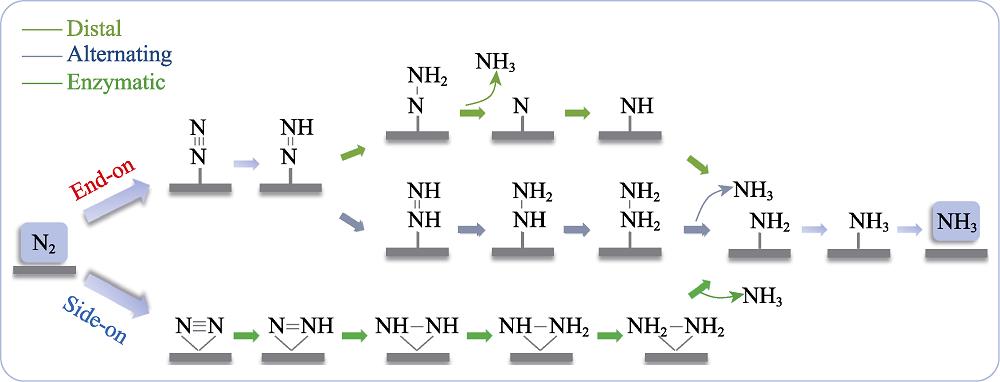
Fig. 3 shows the reaction mechanisms including distal, alternating and enzymatic mechanisms[35,39-40]. The end- on adsorption of nitrogen molecule prefers a distal or alternating mechanism. In distal mechanism, the pair of electrons continuously attacks the nitrogen atom farthest from the catalyst's surface, releasing an ammonia molecule, which then attacks a second nitrogen atom to form another ammonia molecule. In alternating mechanism, the hydrogenation step alternates between two nitrogen atoms on the surface of the catalyst. Nitrogen molecules adsorbed by side-on manner can be reduced to ammonia by enzymatic mechanism, in which two nitrogen atoms alternately hydrogenated on the catalyst surface by proton-electron pairs and release the first ammonia molecule, thereby forming the second one. The free energy changes ΔG of the elementary steps of NiN4/Cr, NiN4/Mo and NiN4/Ta are summarized in Table S5 in the supporting materials.
Fig. 4 gives the free energy profile of NiN4/Cr combination as an illustration. Wherein, the free energy of the *N2 end-on adsorption is downhill by 0.41 eV indicating its spontaneity. In distal mechanism, the free energy of the *N-NH formation is uphill by 0.98 eV. In the second protonation step, the exothermic character of *N-NH2 formation is observed with the ΔG of -0.28 eV. Subsequently, the release of the first NH3 requires overcoming 0.17 eV of energy. The second *NH3 is formed by the *N consecutive reduction where *NH, *NH2 and *NH3 formation is downhill trend with the ΔG of -1.08, -1.09 and -0.23 eV. Finally, desorption of the second *NH3 is hindered by the endothermic ΔG of 1.04 eV. Noteworthy, NH4+ reacted from *NH3 protonation would be desorbed easily with aid of the solution[4]. Therefore, the elementary step of the *NH3 desorption is not a problematic obstacle in NRR[41]. Herein, the PDS in the distal pathway is located at the first protonation with the ΔGmax of 0.98 eV. Analogously, ΔG of the first protonation step via the alternating mechanism is uphill by 0.98 eV. In the second protonation step, the endothermic character of *NH-NH formation is observed with the ΔG of 0.05 eV, indicating the stability of *N-NH structure. Later, an exothermic trend was found in the following protonation, with ΔG values of -0.31, -0.25, -1.29 and -0.71 eV, respectively. Herein, the PDS is also identified at the first protonation for the alternating mechanism with the same ΔGmax of 0.98 eV. Furthermore, for enzymatic mechanism, the free energy of the *N2 side-on adsorption decreased by 0.10 eV. Subsequently, the formation of *N-*NH and *NH-*NH requires overcoming 0.57 and 0.16 eV of energy. In the following protonation process, the downhill trends are revealed by the corresponding ΔG of -0.56, -0.12, -1.51 and -0.38 eV, respectively. Herein, the PDS is located at the first protonation of *N-*NH formation with the value of 0.57 eV. In order to overcome the thermodynamic barrier, the applied U is shifted to 0.98, 0.98 and 0.57 V for distal, alternating and enzymatic mechanism, respectively. The lower onset potential corresponds to the better activity. Therefore, the preferred mechanism of NRR on NiN4/Cr is the enzymatic pathway. Herein, it is noteworthy that our discussion is based on the abundant proton in the acid solution. However, the efficiency of aqueous NRR is complicatedly dependent on the proton supply. The proton-poor neutral/alkaline solutions are generally used to lower the proton accessibility for a suppressed HER in order to improve the NRR efficiency. On the other hand, the limited proton from water splitting also deteriorates NH3 formation since NRR is essentially related to the hydrogenation reactions. Therefore, the variation of the NRR performance caused by different pH solution is still under the debate[42⇓-44].
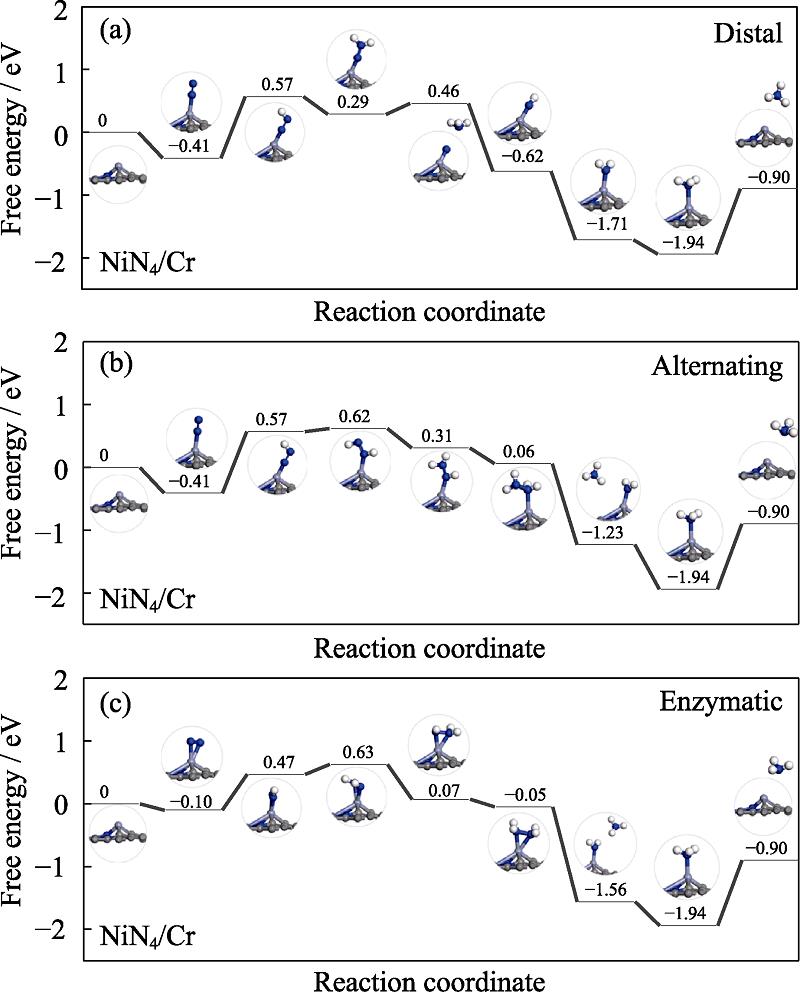
图 4.
Fig. 4. Free energy diagrams and the corresponding configuration of the NRR intermediates on NiN4/Cr NRR mechanisms are (a) distal, (b) alternating and (c) enzymatic
The free energy profiles of NiN4/Mo and NiN4/Ta are presented in Fig. S2 and Fig. S3 in the supporting materials, respectively. As for the former, the PDS are preserved at the first protonation step with the ΔGmax of 0.92, 0.92 and 0.60 eV, respectively. Therefore, NRR follows the enzymatic pathway on NiN4/Mo with the overpotential of 0.60 V. As for the latter, the PDS is the first protonation step for the distal and alternating pathway and the ΔGmax of 0.69 eV, meanwhile the high ΔGmax is located at the *NH2-*NH2 formation with the value of 0.58 eV for the enzymatic pathway. However, the enzymatic pathway of NiN4/Ta is unfavorable considering the endothermic character of the *N-*N adsorption. Therefore, the preferred mechanism of NRR on NiN4/Ta is the distal or alternating. According to the above analysis, ∆Gmax is ordered by NiN4/Cr (0.57 eV)
In order to further reveal the catalytic effect, the Mulliken charge analysis was performed. In line with previous reports[40,45⇓-47], there are three moieties, including moiety 1 (the graphene substrate), moiety 2 (active center) and moiety 3 (NRR intermediates). Fig. 5 presents the charge variation of NiN4/Cr, NiN4/Mo and NiN4/Ta during the protonation steps. The charge transfer is observed between the adsorbent and the substrate, in consistent with previous reports[45]. Besides, Fig. 5(d) monitors the N-N bond lengths of the NRR intermediates. Therein, the stretch of N-N bond is obvious, indicating that the protonation continuously activates the inert N-N bonds and leads to the energetic feasibility of the N2-to-NH3 conversion under the mild condition.
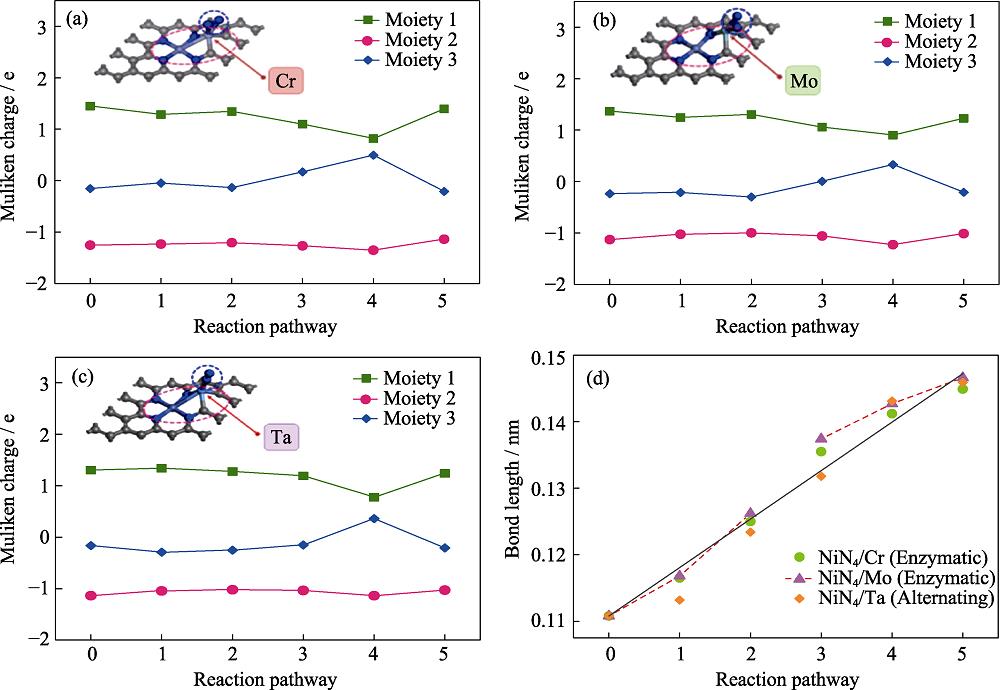
图 5.
Fig. 5. (a-c) Charge variation of the three moieties along the optimal pathway and (d) N-N bond length change in NRR along preferred pathwayMoieties 1, 2, 3 represent the graphene substrate, active center, and NRR intermediates, respectively
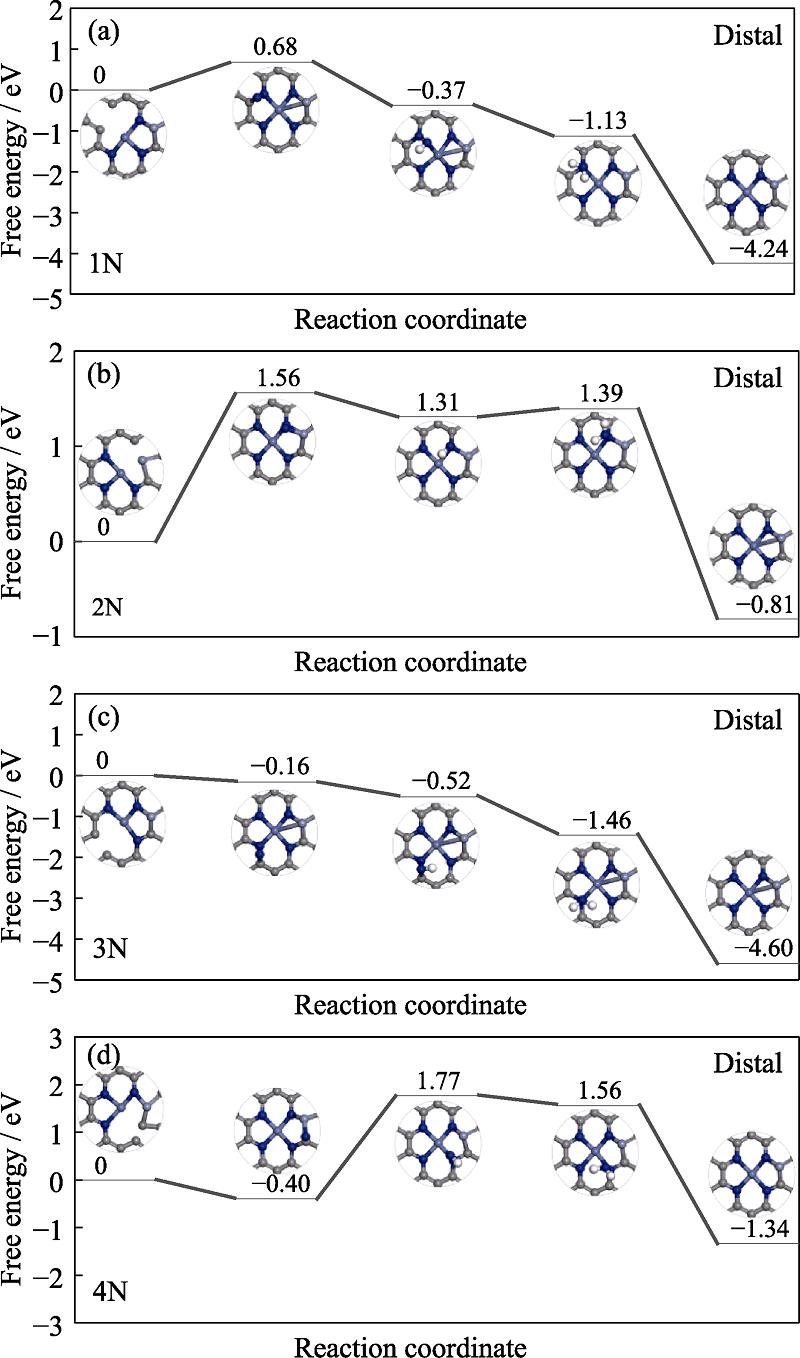
In the end, it is noteworthy that the N vacancy would be created during the reduction environment and act as the active site, besides the transition metal site discussed above. Herein, we further consider the role of nitrogen vacancy. Fig. S7 in the supporting materials presents the corresponding free energy profiles of N2-to-NH3 conversion. For convenience, four different N vacancies are donated as 1N, 2N, 3N and 4N, respectively. The results reveal that the 1N and 2N sites are unable to capture N2 molecule due to the endothermic character meanwhile the first protonation on the 4N site is energetically limited. Differently, the free energy profile of 3N site is continuously declined, demonstrating its feasibility to boost N2-to-NH3 conversion. Therefore, the 3N vacancy would act as an active site for ammonia synthesis. However, according to the reaction of N4*+3(H++e-)→ N3*+NH3, the formation energy of 3N site is 3.70 eV. It is energy-demanding process, implying the difficulty for the vacancy formation. It stems from the strong interaction between N atom and its surrounding, as reflected by the binding energy with the value of 9.91 eV. Furthermore, Fig. S8 in the supporting materials reveals that the nitrogen atom is accessible for one hydrogen atom but it is unable to capture more hydrogen atoms. Therefore, the creation of the N vacancy via protonation steps is energetically adverse. Considering the mentioned discussion, we do not further devote our attention on the N vacancy.
3 Conclusions
In summary, the NRR performance of the TM1N4/TM2 embedded graphene is systematically investigated by means of density functional theory calculation. Considering activity and stability, our results reveal that NiN4/Cr, NiN4/Mo and NiN4/Ta exhibit outstanding performance toward NRR, being promising alternatives to the commercial Ru(0001). Herein, ∆Gmax is ordered by NiN4/Cr (0.57 eV)
8 Supporting materials
Supporting materials related to this article can be found at
9 Supporting Materials:
WU Jing1, YU Libing1, LIU Shuaishuai1, HUANG Qiuyan1, JIANG Shanshan1, Anton Matveev2, WANG Lianli3, SONG Erhong4, XIAO Beibei1
(1. School of Energy and Power Engineering, Jiangsu University of Science and Technology, Zhenjiang 212003, China; 2. National Research Ogarev Mordovia State University, Saransk 430005, Russia; 3. School of Materials Science and Engineering, Xi’an University of Science and Technology, Xi’an 710054, China; 4. The State Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, China)
表 1.
Adsorption energies Eads on Mn1N4/TM2 (Eads in eV)
Table 1.
Adsorption energies Eads on Mn1N4/TM2 (Eads in eV)
|
表 2.
Adsorption energies Eads on Fe1N4/TM2 (Eads in eV)
Table 2.
Adsorption energies Eads on Fe1N4/TM2 (Eads in eV)
|
表 3.
Adsorption energies Eads on Co1N4/TM2 (Eads in eV)
Table 3.
Adsorption energies Eads on Co1N4/TM2 (Eads in eV)
|
表 4.
Adsorption energies Eads on Ni1N4/TM2 (Eads in eV)
Table 4.
Adsorption energies Eads on Ni1N4/TM2 (Eads in eV)
|
表 5.
Free energy change ΔG (ΔG in eV), Ri stands for the ith protonation step
Table 5.
Free energy change ΔG (ΔG in eV), Ri stands for the ith protonation step
|
表 6.
Potential determining step and its free energy change ΔGmax(ΔGmax in eV)
Table 6.
Potential determining step and its free energy change ΔGmax(ΔGmax in eV)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
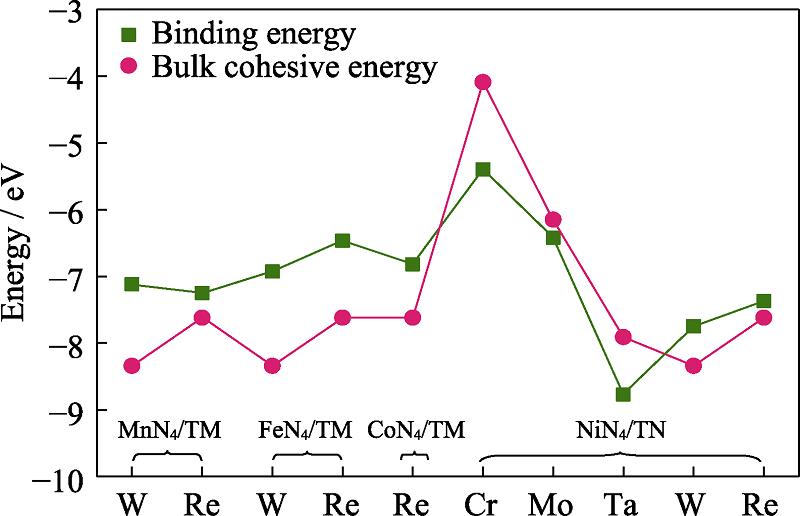
图 6.
Fig. 6. Comparison of binding energy and bulk cohesive energy of the selected complexes
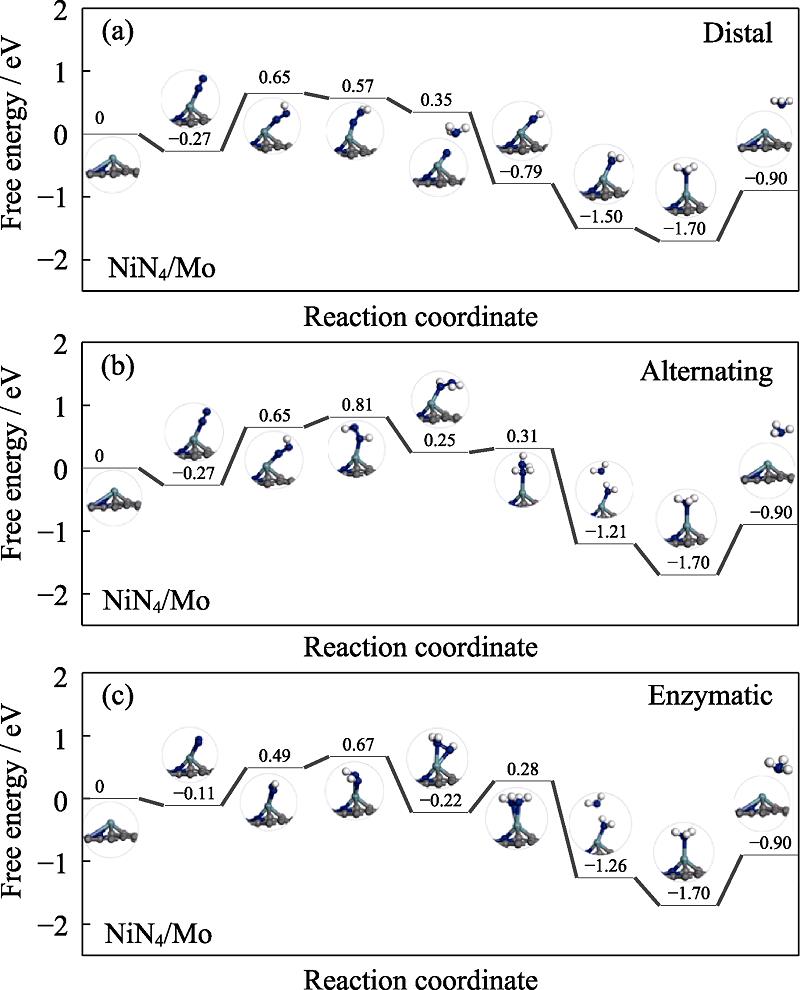
图 7.
Fig. 7. Free energy diagrams and the corresponding configuration of the NRR intermediates on NiN4/Mo NRR mechanisms are (a) distal, (b) alternating, and (c) enzymatic, respectively
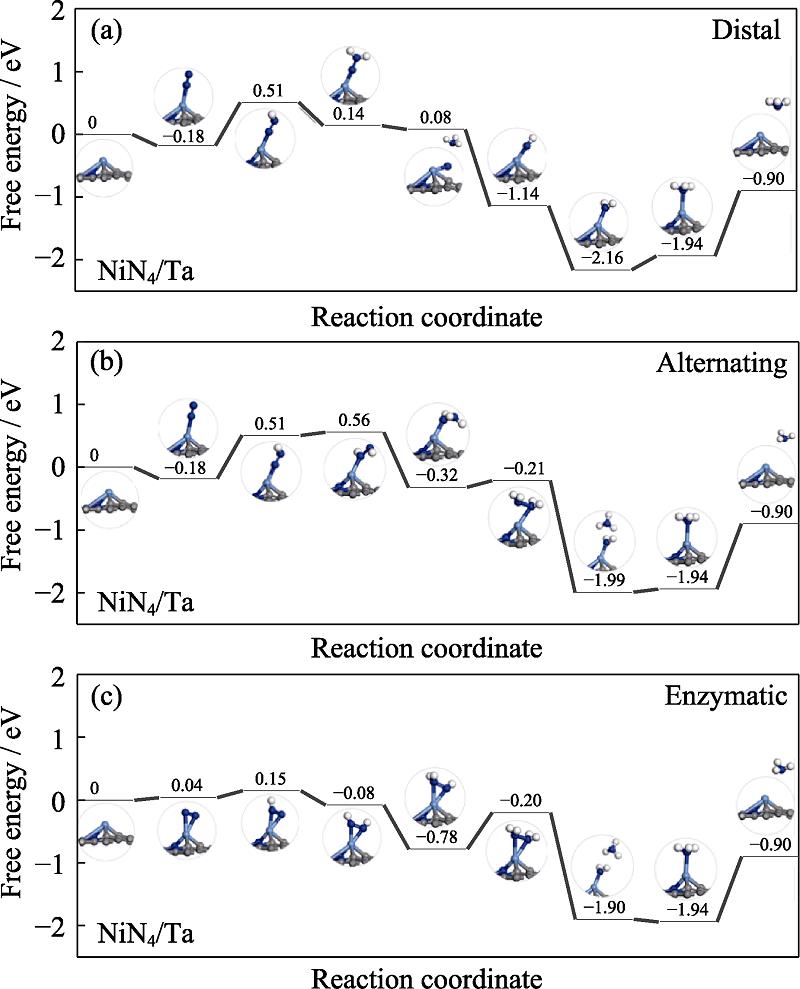
图 8.
Fig. 8. Free energy diagrams and the corresponding configuration of the NRR intermediates on NiN4/Ta NRR mechanisms are (a) distal, (b) alternating, and (c) enzymatic, respectively
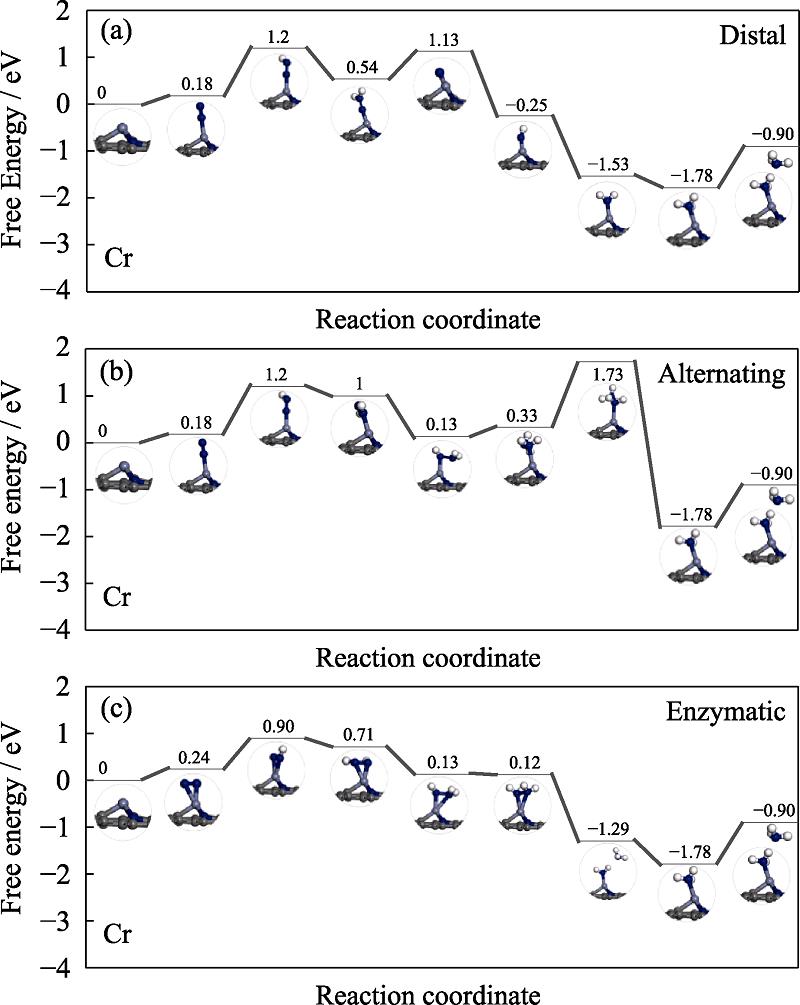
图 9.
Fig. 9. Free energy diagrams and the corresponding configuration of the NRR intermediates on Cr embedded nitrogen functionalized grapheneNRR mechanisms are (a) distal, (b) alternating, and (c) enzymatic, respectively
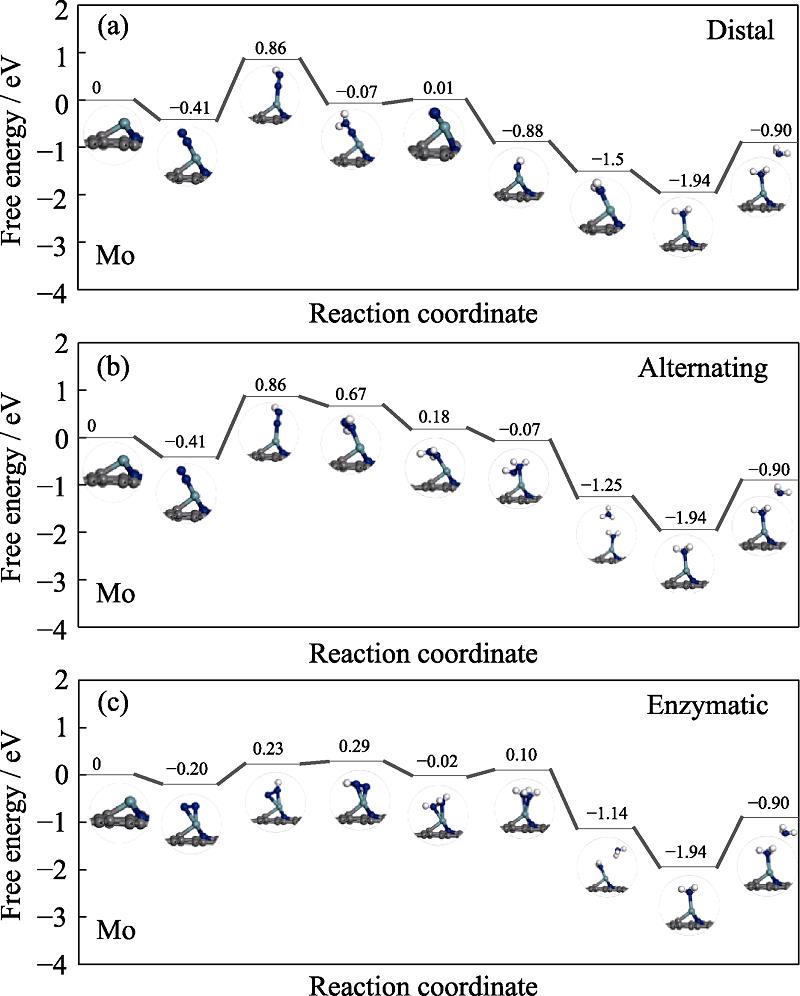
图 10.
Fig. 10. Free energy diagrams and the corresponding configuration of the NRR intermediates on Mo embedded nitrogen functionalized grapheneNRR mechanisms are (a) distal, (b) alternating, and (c) enzymatic, respectively
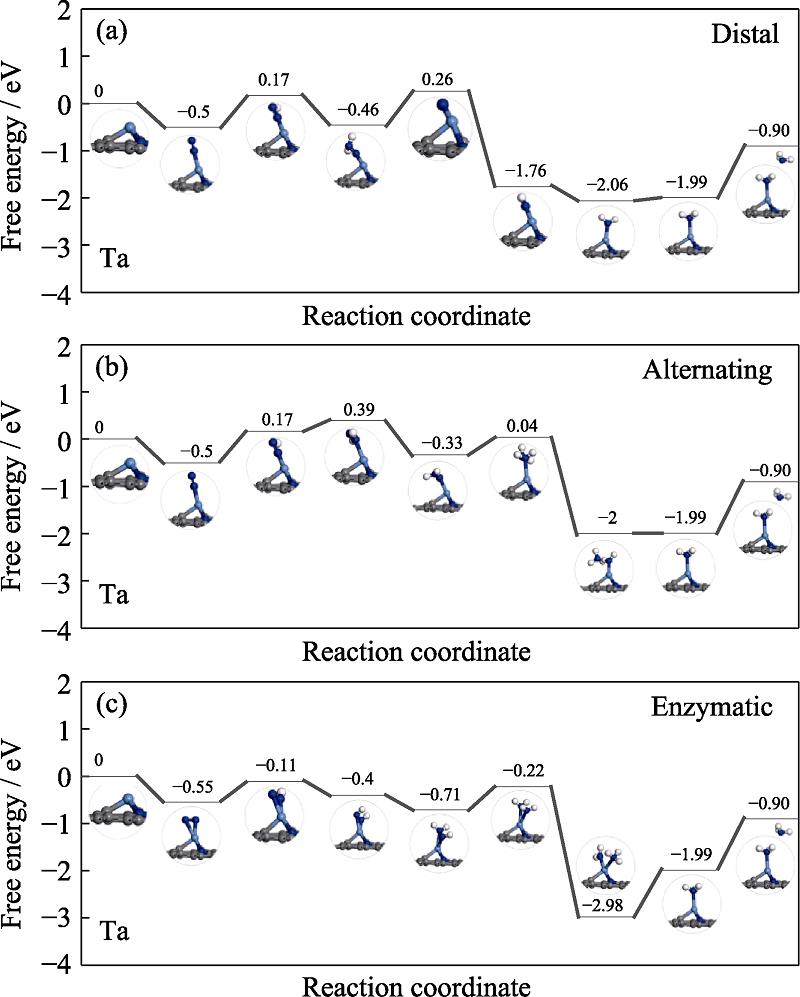
图 11.
Fig. 11. Free energy diagrams and the corresponding configuration of the NRR intermediates on Ta embedded nitrogen functionalized grapheneNRR mechanisms are (a) distal, (b) alternating, and (c) enzymatic, respectively
[1] WANG Y, JIA K, PAN Q, et al. Boron-doped TiO2 for efficient electrocatalytic N2 fixation to NH3 at ambient conditions[J]. ACS Sustain. Chem. Engineer., 2018: 117-122.
[17] RIYAZ M, GOEL N. Single-atom catalysis using chromium embedded in divacant graphene for conversion of dinitrogen to ammonia[J]. ChemPhysChem, 2019: 1954-1959.
[19] ZHENG X N, YAO Y, WANG Y, et al. Tuning the electronic structure of transition metals embedded in nitrogen-doped graphene for electrocatalytic nitrogen reduction: a first-principles study[J]. Nanoscale, 2020: 9696-9707.
[20] ZHOU Y, SONG E H, CHEN W, et al. Dual-metal interbonding as the chemical facilitator for single-atom dispersions[J]. Adv. Mater., 2020: e2003484.
[21] DELLY B. An all-electron numerical method for solving the local density functional for polyatomic molecules[J]. J. Chem. Phys., 1990: 508-517.
[22] DELLY B. From molecules to solids with the DMol3 approach[J]. J. Chem. Phys., 2000: 7756-7764.
[23] OERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys. Rev. Lett., 1996: 3865-3868.
[24] DELLY B. Hardness conserving semilocal pseudopotentials[J]. Phys. Rev. B, 2002: 155125.
[33] YIN J, FANG Q H, LI Y X, et al. Ni-C-N nanosheets as catalyst for hydrogen evolution reaction[J]. J. Am. Chem. Soc., 2016: 14546-14549.
[35] LING C Y, OUYANG Y X, LI Q, et al. A general two-step strategy-based high-throughput screening of single atom catalysts for nitrogen fixation[J]. Small Methods, 2019: 1-8.
[41] QIU W B, XIE X Y, QIU J D, et al. High-performance artificial nitrogen fixation at ambient conditions using a metal-free electrocatalyst[J]. Nat. Commun., 2018: 3485.
[45] ZHAO J X, CHEN Z F. Single Mo atom supported on defective boron nitride monolayer as an efficient electrocatalyst for nitrogen fixation: a computational study[J]. J. Am. Chem. Soc., 2017: 12480-12487.
Article Outline
吴静, 余立兵, 刘帅帅, 黄秋艳, 姜姗姗, ANTON Matveev, 王连莉, 宋二红, 肖蓓蓓. NiN4/Cr修饰的石墨烯电化学固氮电极[J]. 无机材料学报, 2022, 37(10): 1141. Jing WU, Libing YU, Shuaishuai LIU, Qiuyan HUANG, Shanshan JIANG, Matveev ANTON, Lianli WANG, Erhong SONG, Beibei XIAO.