基于多光子聚合微笼阵列的单细胞捕获方法  下载: 691次封面文章
下载: 691次封面文章
The study of single cell is of great significance in the fields of cell heterogeneity, genetic metabolism, genetic engineering, and toxicity detection. To identify the functional characteristics of individual cells, individual cell capture must first be achieved. However, most trapping methods require a constant force to keep the cell trapped. When the force decreases or disappears, the cells easily revert to a disordered state, which is very harmful to subsequent characterization and analysis. Therefore, a method for capturing individual cells stably without the use of constant external forces is expected to open up a new avenue for single cell research. This paper proposes a method of rapid capture of single cell during the self-assembly process of micropillars based on femtosecond laser multiphoton processing technology and the principle of capillary force self-assembly. It has fast capture, convenient operation, and a broad application range, and has a lot of potential in bioengineering, drug analysis, and other fields.
High aspect ratio micropillars were prepared by single-pulse femtosecond laser multiphoton polymerization. The height and space of the micropillars can be adjusted by moving the micro/nano translation stages vertically and horizontally. The laser-processed sample was developed upside down by a developer for 6 min to remove the photoresist in the unprocessed area. After the sample was developed, it was washed in isopropanol solution for 10 min to remove any residual developer. To prevent the self-assembly of the sample after developing and cleaning, we put it into a PBS solution immediately. The sample was sealed and degassed for 8 h before being disinfected. Drop the cell solution directly above the micropillar arrays with a density of 1.2×105 cell/mL. After seeding cells, the sample was placed in the cell incubator for 2 h to allow the cells to fully adhere to the Petri dish and fall into the bottom of the micropillar gap. Subsequently, the uncapped cells were washed off with trypsin, and the sample was processed using living dead staining. Using a pipette gun, remove the liquid and allow the residual solution to evaporate naturally. The cells between the micropillars were captured during the self-assembly process of micropillars.
The micropillar structure with a high aspect ratio has a large diameter at the bottom and gradually shrinks to the top, showing a conical shape (Fig. 1). The bottom diameter of the micropillar gradually increases as the height and laser power increase. By shortening the distance between micropillars, increasing the distance between self-assembly structures, and reasonably adjusting the height of micropillars, the micropillars close to each other can be self-assembled based on capillary force, to realize the high-efficiency preparation of largescale three-dimensional (3D) complex patterned self-assembly structures (Fig. 2). The experiment of micropillars’ self-assembly driven by capillary force to capture microspheres shows that the micropillars can still be self-assembled into microcage structures when there are particles in the micropillar gap (Fig. 3). Based on the above methods, a single cell array capture experiment was carried out. The results of fluorescence imaging and scanning electron microscope (SEM) images show that this method can realize high-throughput in situ capture of single cell array simply and efficiently (Fig. 5). Additionally, the cell capture experiment of microcage composed of a different number of micropillars provides a relatively simple method for sorting, capturing, and in situ observation of cells of different sizes (Fig. 6). The four micropillar microscage can only capture cell with similar diameter of the microcage, providing a new method for cells sorting. The six micropillar microscage can capture different sizes of cells, and single cell analysis experiment can be carried out by the micropillars’ gap. The eight micropillar microscage can strictly restrict the captured cell well in the microcage and is expected to be used in domain-limited growth characteristics research of single cell.
To meet the application requirements of high throughput single cell capture, a domain-limited passive capture method of single cell arrays based on self-assembly of a micropillar array is proposed. Based on femtosecond laser single pulse multiphoton polymerization technology and the capillary force self-assembly principle, this method realizes the high-throughput in situ capture of the MCF-7 single cell array. Using femtosecond laser single pulse multiphoton polymerization to achieve the high-efficiency preparation of micropillar arrays with a high aspect ratio. By optimizing the spacing and height of micropillars as well as largescale path planning, high throughput capillary force self-assembly of a largescale 3D complex microcage structure was achieved. The in situ capture of a single cell array based on capillary force self-assembly was successfully realized by matching the structural parameters of the microcage with the size of the cells. Different from the traditional single cell capture methods, such as single cell orientation capture method previously proposed by us, this method provides a relatively simple and efficient method for the sorting, capture, and in situ observation of cells with different sizes without the need for continuous additional external force. It has various application prospects in the fields of bioengineering, pharmaceutical analysis, and other domains.
1 引言
单细胞分析对于细胞异质学[1-2]、遗传代谢、基因工程及毒性检测等方面的研究具有重要意义[3-4],而单细胞分析的前提是捕获单个细胞并形成单细胞阵列。目前,常用的细胞捕获方法大多数需要与微流控技术相结合,比如单光束激光法[5-6]、介电泳[7-9]、声镊[10-11]及磁镊[12-14]。介电泳捕获细胞的原理是将细胞在非均匀电场中极化,从而使其在介电泳力的作用下运动或者被势阱限制。声镊则是利用超声驻波产生声压,实现对单细胞的操纵和捕获。显然,利用上述方法捕获细胞时,需要外加电场、磁场等来维持细胞被捕获的状态,一旦取消外加物理场的作用,细胞很容易回到无序状态,不利于后续进一步的表征和分析。因此,发展一种类似于微结构的方式来实现捕获后细胞的固定、培养和观测,是单细胞分析亟待解决的难题。
飞秒激光加工技术具有真三维、非线性吸收和高分辨率等特点[15-17],不仅可以制备超疏水表面[18]、浸润性表面[19-20]、高精密微孔[21-22],还可以灵活地在各种透明聚合材料中制造具有超高分辨率的复杂微结构[23-25]。因此,利用飞秒激光加工技术实现对单细胞的原位捕获是一种重要方法。Parmeggiani研究小组[26]利用激光直写技术加工出了一种能够捕捉微粒的微手,它可以通过光学照明远程控制来抓取小颗粒,但是由于操作方式复杂,难以实现高通量捕获。许兵[27]利用飞秒激光双光子光刻技术,通过在单个目标颗粒周围加工一圈微柱的方式来捕获颗粒,实现了对特定单目标颗粒的捕获;但这种方法需要将捕获目标混入到光刻胶中,不适合用于单细胞的高通量捕获。所查资料显示,目前缺少一种既可以高效率制备微结构,又可以实现细胞高通量快速捕获的方法。
本文发展了一种基于微柱阵列自组装的单细胞捕获方法,实现了人乳腺癌细胞(MCF-7细胞)的原位捕获。这种方法基于飞秒激光单脉冲多光子聚合原理并结合毛细力自组装有效实现了单细胞的限域被动捕获。本团队首先研究了加工参数对微柱阵列的影响,然后优化微柱的高度和间距,实现微结构与MCF-7细胞尺寸的匹配,最后通过细胞原位捕获实验得到了4×4的MCF-7单细胞阵列。对本文所提方法进行进一步优化,可有效产生大规模的单细胞阵列。本文方法在单细胞水平特异性分析和药物筛选等领域具有巨大的应用潜力。
2 微结构阵列的高通量制备
2.1 单脉冲多光子聚合制备微柱
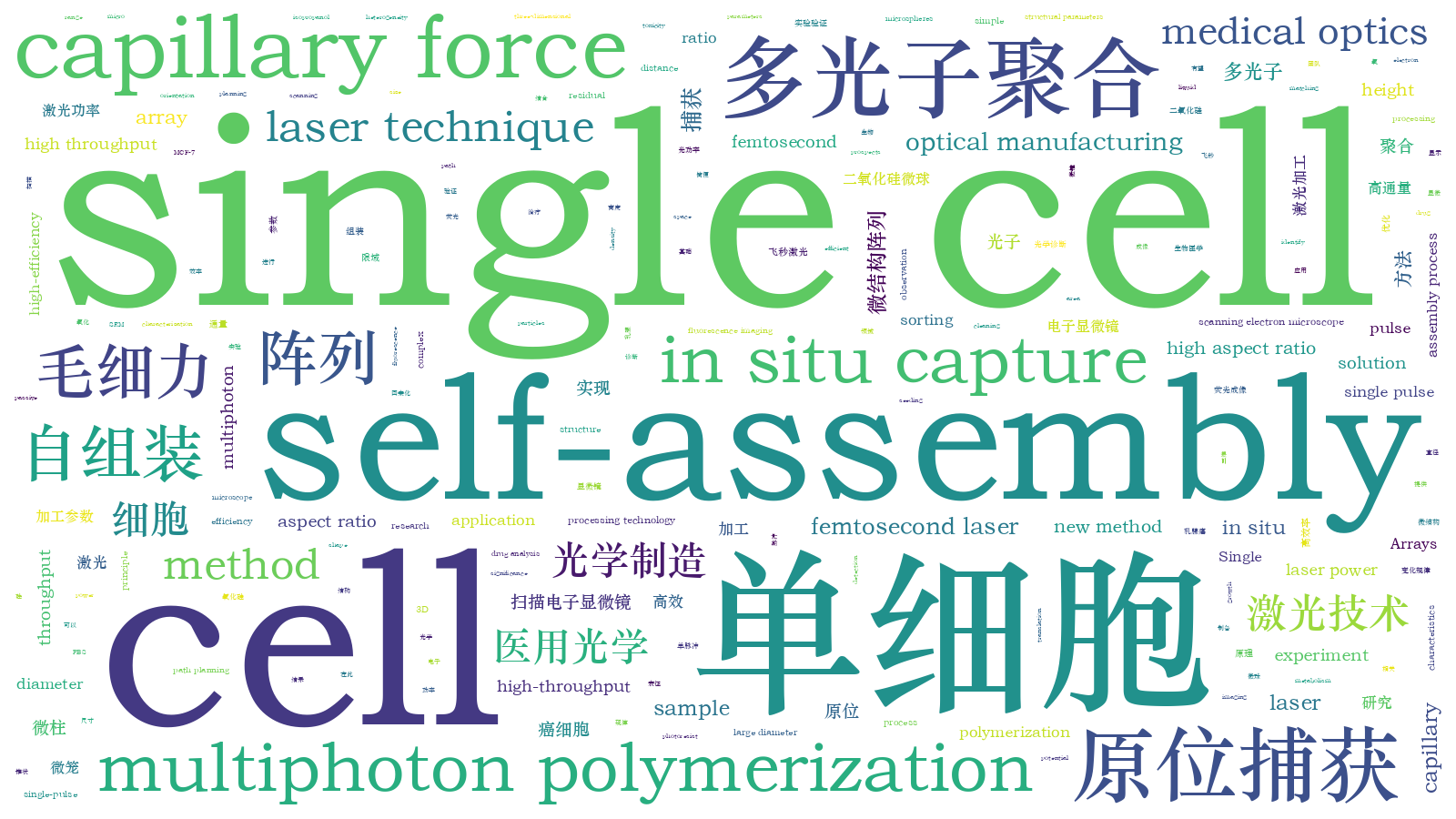
飞秒激光多光子聚合加工系统主要由飞秒激光光路系统、X-Y-Z三维精密运动平台、CCD成像模块和软件控制模块组成。本文所用飞秒激光器的波长为1030 nm,重复频率为200 kHz,脉冲宽度为217 fs。针对多光子聚合轴向加工效率较低的问题,本团队采用前期提出的高纵宽比的聚焦光斑进行微柱的单脉冲多光子聚合加工[28],实验原理如
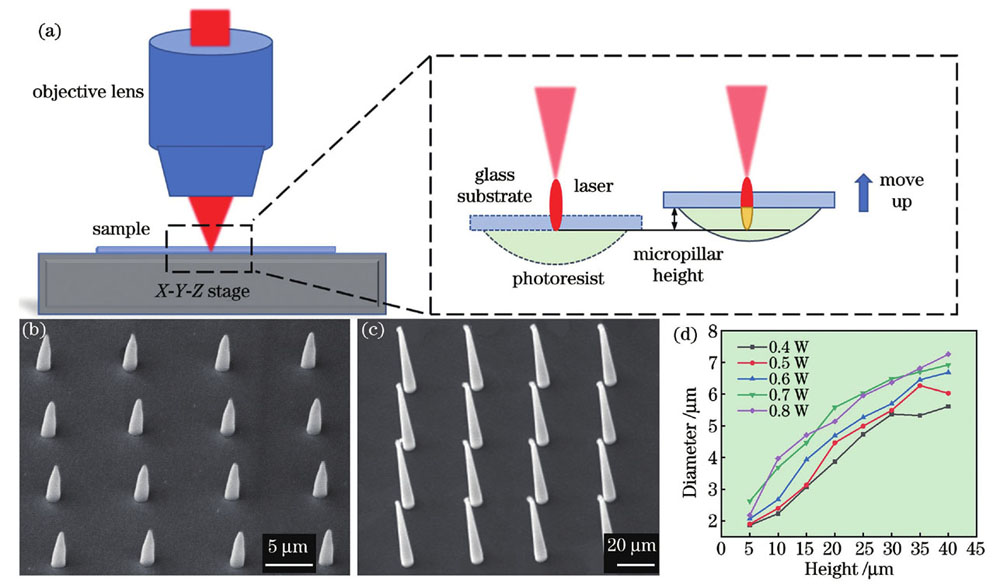
图 1. 单脉冲多光子聚合实验原理以及加工的微柱阵列。(a)单脉冲多光子聚合原理示意图,右边虚线框内为倒置样品加工过程的细节放大图,红色椭圆形区域代表聚焦光斑的能量分布,黄色区域表示光刻胶中由聚焦光斑引起的多光子聚合区域;(b)(c)单脉冲多光子聚合加工得到的高度为5 μm和40 μm的4×4微柱阵列;(d)入射激光功率为0.4~0.8 W时,微柱 直径随高度的变化曲线
Fig. 1. Schematic illustrations of single-femtosecond-laser-pulse-based multiphoton polymerization principle and processed micropillars arrays. (a) Schematic illustrations of single-femtosecond-laser-pulse-based multiphoton polymerization principle, where dotted box on the right shows an enlarged view of the processing of inverted sample, red ellipse area represents energy distribution of laser focal spot, and yellow area represents multiphoton polymerized region caused by the corresponding focal spot in the photoresist; (b)(c) 4×4 micropillar arrays with height of 5 μm and 40 μm processed by single-femtosecond-laser-pulse-based multiphoton polymerization; (d) relationship between micropillar diameter and height when laser power ranges from 0.4 W to 0.8 W
基于上述加工原理对单脉冲多光子聚合微柱的加工参数进行研究。在实验中,通过移动纳米位移平台对样品高度进行调节,进而实现对微柱高度的精确控制。利用扫描电子显微镜(SEM)表征微柱的高度和直径。
2.2 毛细力自组装微结构阵列的制备方法
当微柱之间的距离减小时,随着显影液的挥发,处于液-气界面的两个相邻微柱,其顶端在毛细力作用下有相互靠近的趋势。当相邻微柱间的距离足够近时,微柱受到的静毛细力大于弹性恢复力,微柱间发生毛细力自组装,形成微柱簇结构。值得注意的是,微柱间毛细力的大小既与微柱间距有关,也与微柱高度有关[29]。在微柱间距一定的情况下,微柱受到的弹性恢复力随着微柱高度的增大逐渐减小,当微柱受到的弹性恢复力小于静毛细力时,微柱间同样会发生毛细力自组装。当微柱所受静毛细力与弹性恢复力处于临界状态时,液体挥发过程中发生的不稳定自组装的实验结果如
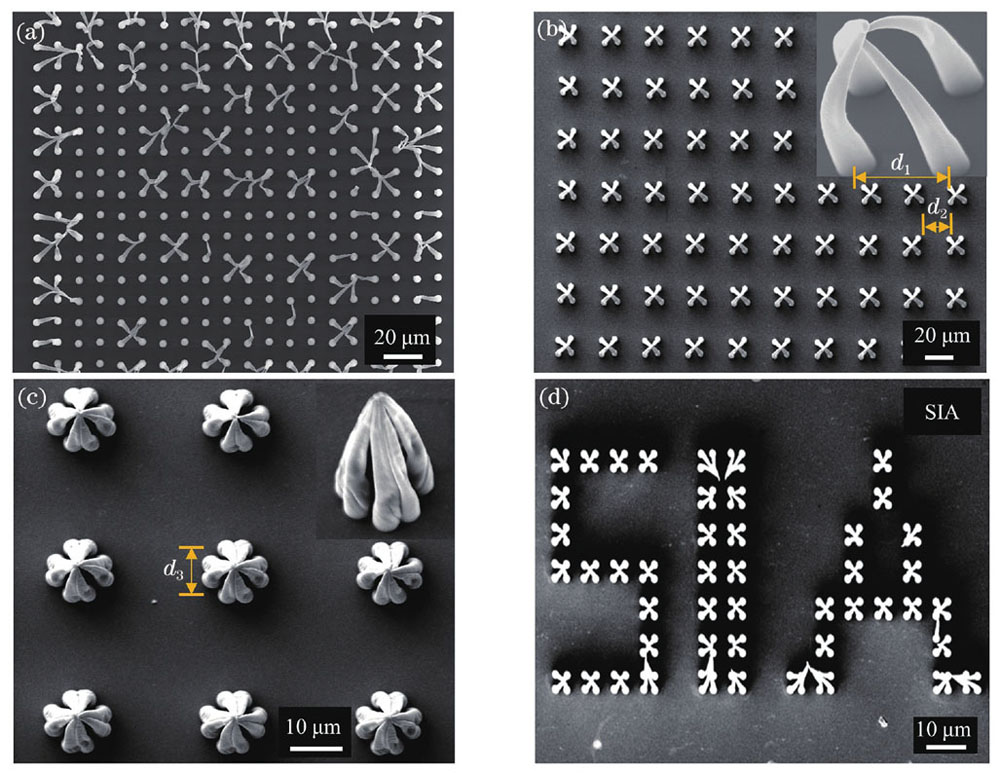
图 2. 基于毛细力自组装的图案化微结构阵列,其中加工微柱的激光功率均为0.4 W。(a)临界状态时微柱不稳定自组装结果;(b)由4个微柱自组装形成的微笼结构阵列,右上角为单个微笼的侧视放大图;(c)由8个微柱自组装形成的“四叶草”微结构阵列,右上角为侧视放大图;(d)以(b)中自组装结构为子单元加工的“SIA”图案化结构
Fig. 2. Patterned microstructural arrays based on capillary force self-assembly. (a) Unstable self-assembly of micropillars in critical state; (b) microcage arrays assembled by four micropillars, the enlarged view of the microcage structure is shown at upper right corner; (c)“four-leaf clover”microstructure array assembled by eight micropillars, the enlarged view is shown the upper right corner; (d)“SIA”patterned structure consisted by the self-assembled structure in (b) as a subunit
鉴于飞秒激光多光子聚合的高空间选择性,将其与本文所提单脉冲微柱多光子聚合方法结合,可以实现多样化三维自组装结构的高通量加工。
3 单细胞阵列的原位捕获
3.1 微球的原位捕获
在开展基于自组装原理的细胞原位捕获实验之前,首先通过微球原位捕获实验对微柱间隙存在其他物体时微柱的自组装能力进行实验验证。微柱间隙中的物体选取的是直径为10 μm的二氧化硅微球,确保其可在无水乙醇溶液中发生沉降。采用的无水乙醇可在空气中快速挥发,缩短了自组装所需时间。
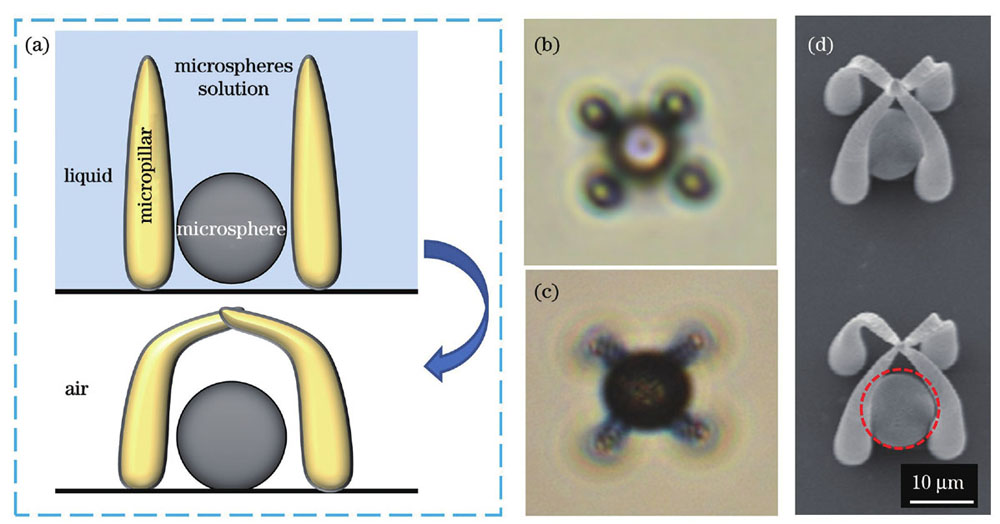
图 3. 基于毛细力自组装的微球原位捕获。(a)捕获微球的过程示意图;(b)在液体环境中,微球落入微结构的光学图像;(c)在空气环境中,微球被捕获后的光学图像;(d)捕获微球的SEM图,红色虚线圆环中是被捕获的微球
Fig. 3. In situ capture of microspheres based on capillary force self-assembly. (a) Diagram of the process of capturing microspheres; (b) optical image of microsphere falling into microstructures in liquid; (c) optical image of captured microspheres in the air; (d) SEM images of microspheres captured, where the captured microsphere is in the red dotted ring
3.2 单细胞阵列的原位高通量捕获
在上述实验的基础上,开展基于毛细力自组装的单细胞阵列的原位捕获实验。与微球相比,MCF-7细胞较为柔软而且不同细胞的形态、尺寸差异较大。考虑到细胞接种过程中的大部分细胞呈球状,更容易落入特定尺寸的微笼中,因此,本团队在微球捕获实验的基础上对实验步骤进行进一步优化,使其适用于柔软、易变形细胞的原位捕获。单细胞阵列原位捕获实验流程如
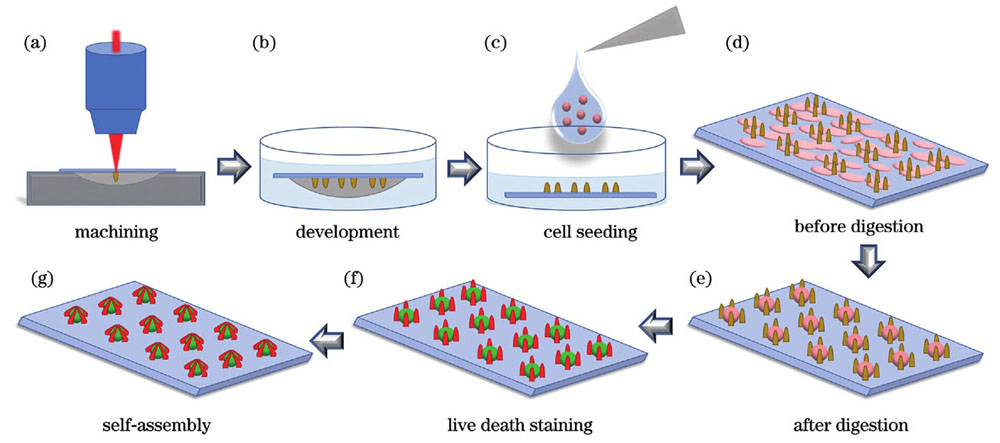
图 4. 单细胞阵列原位捕获实验步骤示意图。(a)微结构阵列的扫描加工;(b)微结构倒置显影;(c)细胞接种到微结构样本上;(d)细胞贴壁到样本表面;(e)对样本进行细胞消化处理后得到的单细胞阵列;(f)单细胞阵列活死染色;(g)毛细力自组装实现单细胞阵列的原位捕获
Fig. 4. Schematics of single cell array in situ capture experiment procedure. (a) Scanning fabrication of micropillar arrays; (b) inverted development of micropillar structures; (c) cell seeding on microstructural samples; (d) cells adhering to the sample surface; (e) single cell array obtained after cell digestion process; (f) single cell array processed by living dead staining; (g) in situ capture of single cell array by capillary-force-based self-assembly of micropillars
鉴于本文所提方法是一种被动的捕获方式,要求细胞的结构尺寸与微柱自组装形成的微笼结构的尺寸相匹配,因此,首先对消化处理后的MCF-7细胞的直径进行了测量。
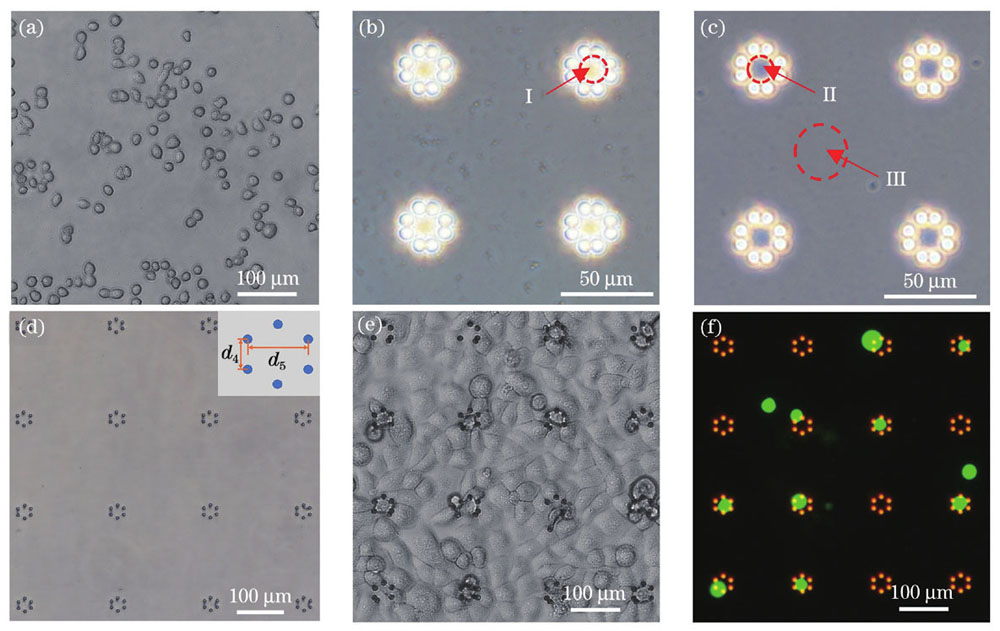
图 5. 微笼阵列的单细胞捕获。(a)MCF-7细胞;(b)(c)除气操作处理前后微结构阵列的光学图像;(d)未接种细胞前,培养液中六微柱微笼阵列的光学图像;(e)接种细胞后,样本表面结构和细胞相对分布的光学图像;(f)消化处理后得到的细胞荧光图像
Fig. 5. Single cell capture in microcage arrays. (a) MCF-7 cells; (b)(c) optical images of microstructure array before and after degassing operation processing; (d) optical image of six micropillars microcage array in culture medium before cell seeding; (e) optical image of sample surface structure and relative distribution of cells after cell seeding; (f) fluorescence images of cells obtained after digestion
在上述实验的基础上,本团队开展了基于毛细力自组装原理的单细胞阵列原位捕获实验。在如
为研究不同自组装微结构的单细胞捕获效果,本团队开展了由不同数量微柱组成的微笼的细胞捕获实验。
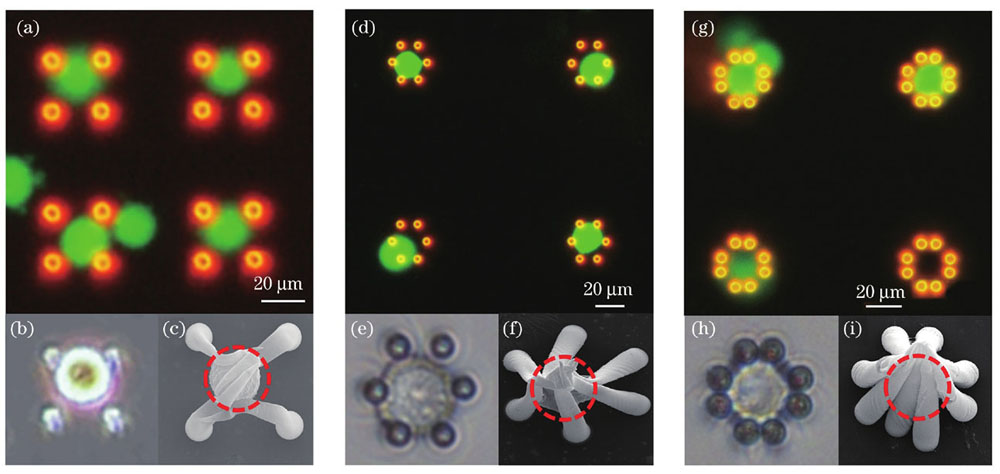
图 6. 细胞原位捕获图。(a)(b)(c)四微柱结构原位捕获细胞的荧光图像、光学图像及SEM图;(d)(e)(f)六微柱结构原位捕获细胞的荧光图像、光学图像及SEM图;(g)(h)(i)八微柱结构原位捕获细胞的荧光图像、光学图像及SEM图
Fig. 6. Cells in situ capture diagram. (a)(b)(c) Fluorescence image, optical image and SEM image of cells in situ capture by four micropillars structures; (d)(e)(f) fluorescence image, optical image and SEM image of cells in situ capture by six micropillars structures; (g)(h)(i) fluorescence image, optical image and SEM image of cells in situ capture by eight micropillars structures
4 结论
针对高通量单细胞捕获的应用需求,本团队基于飞秒激光单脉冲多光子聚合技术,结合毛细力自组装原理,提出了一种基于微柱阵列自组装的单细胞阵列限域被动捕获方法,并采用该方法实现了MCF-7单细胞阵列的高通量原位捕获。利用飞秒激光单脉冲多光子聚合实现了高纵宽比微柱阵列的高效制备;通过微柱间距与高度的优化及其大范围路径规划,实现了大范围三维复杂微笼结构的高通量毛细力自组装;通过微笼结构参数与细胞尺寸的匹配,成功实现了基于毛细力自组装的单细胞阵列的原位捕获。所提方法为不同尺寸细胞的分选、捕获及原位观测提供了一种较为简便的方法,有望应用于生物工程、医药分析等领域。
[1] He D X, Mao A Q, Zheng C B, et al. Aortic heterogeneity across segments and under high fat/salt/glucose conditions at the single cell level[J]. National Science Review, 2020, 7(5): 881-896.
[2] Galler K, Bräutigam K, Große C, et al. Making a big thing of a small cell: recent advances in single cell analysis[J]. The Analyst, 2014, 139(6): 1237-1273.
[3] Schubert C. Single cell analysis: the deepest differences[J]. Nature, 2011, 480(7375): 133-137.
[4] Weaver W M, Tseng P, Kunze A, et al. Advances in high-throughput single cell microtechnologies[J]. Current Opinion in Biotechnology, 2014, 25: 114-123.
[5] Wang X L, Gou X, Chen S X, et al. Cell manipulation tool with combined microwell array and optical tweezers for cell isolation and deposition[J]. Journal of Micromechanics and Microengineering, 2013, 23(7): 075006.
[6] Ozasa K, Won J, Song S, et al. Autonomous oscillation/separation of cell density artificially induced by optical interlink feedback as designed interaction between two isolated microalgae chips[J]. Scientific Reports, 2016, 6: 24602.
[7] Hunt T P, Westervelt R M. Dielectrophoresis tweezers for single cell manipulation[J]. Biomedical Microdevices, 2006, 8(3): 227-230.
[8] Voldman J, Gray M L, Toner M, et al. A microfabrication-based dynamic array cytometer[J]. Analytical Chemistry, 2002, 74(16): 3984-3990.
[9] Wu C H, Chen R F, Liu Y, et al. A planar dielectrophoresis-based chip for high-throughput cell pairing[J]. Lab on a Chip, 2017, 17(23): 4008-4014.
[10] Petersson F, Nilsson A, Holm C, et al. Separation of lipids from blood utilizing ultrasonic standing waves in microfluidic channels[J]. The Analyst, 2004, 129(10): 938-943.
[11] Guo F, Mao Z M, Chen Y C, et al. Three-dimensional manipulation of single cells using surface acoustic waves[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(6): 1522-1527.
[12] Zhao L B, Pan L, Zhang K, et al. Generation of Janus alginate hydrogel particles with magnetic anisotropy for cell encapsulation[J]. Lab on a Chip, 2009, 9(20): 2981-2986.
[13] Nisisako T, Torii T, Takahashi T, et al. Synthesis of monodisperse bicolored Janus particles with electrical anisotropy using a microfluidic co-flow system[J]. Advanced Materials, 2006, 18(9): 1152-1156.
[14] Kang J H, Krause S, Tobin H, et al. A combined micromagnetic-microfluidic device for rapid capture and culture of rare circulating tumor cells[J]. Lab on a Chip, 2012, 12(12): 2175-2181.
[15] Begantsova Y E, Zvagelsky R, Baranov E V, et al. Imidazole-containing photoinitiators for fabrication of sub-micron structures by 3D two-photon polymerization[J]. European Polymer Journal, 2021, 145: 110209.
[16] Yang D, Liu L P, Gong Q H, et al. Rapid two-photon polymerization of an arbitrary 3D microstructure with 3D focal field engineering[J]. Macromolecular Rapid Communications, 2019, 40(8): e1900041.
[17] Zheng Y C, Zhao Y Y, Zheng M L, et al. Cucurbit[7]uril-carbazole two-photon photoinitiators for the fabrication of biocompatible three-dimensional hydrogel scaffolds by laser direct writing in aqueous solutions[J]. ACS Applied Materials & Interfaces, 2019, 11(2): 1782-1789.
[18] 白雪, 陈烽. 飞秒激光制备超疏水表面的研究进展[J]. 光学学报, 2021, 41(1): 0114003.
[19] 吴志鹏, 银恺, 吴俊瑞, 等. 飞秒激光微纳制造水下气体浸润性表面[J]. 激光与光电子学进展, 2020, 57(11): 111418.
[20] 张明池, 刘子源, 潘宁, 等. 飞秒激光制备不锈钢微纳结构表面的润湿机制研究[J]. 中国激光, 2021, 48(18): 1802001.
[21] 郭敏超, 王明娣, 张胜江, 等. FR-4覆铜板飞秒激光微孔加工工艺研究[J]. 中国激光, 2020, 47(12): 1202008.
[22] 王解, 赵宗晨, 江超, 等. 飞秒激光在单模光纤中精密加工微孔及其传感应用[J]. 激光与光电子学进展, 2020, 57(11): 111425.
[23] Zhang S, Li S G, Wan X Y, et al. Ultrafast, high-resolution and large-size three-dimensional structure manufacturing through high-efficiency two-photon polymerization initiators[J]. Additive Manufacturing, 2021, 47: 102358.
[24] Faraji R Z, Prewett P D, Davies G J. High-resolution two-photon polymerization: the most versatile technique for the fabrication of microneedle arrays[J]. Microsystems & Nanoengineering, 2021, 7: 71.
[25] Cardenas-Benitez B, Eschenbaum C, Mager D, et al. Pyrolysis-induced shrinking of three-dimensional structures fabricated by two-photon polymerization: experiment and theoretical model[J]. Microsystems & Nanoengineering, 2019, 5: 38.
[26] Martella D, Nocentini S, Nuzhdin D, et al. Photonic microhand with autonomous action[J]. Advanced Materials, 2017, 29(42): 1704047.
[27] 许兵. 功能化微流控芯片的飞秒激光高效集成技术研究[D]. 合肥:中国科学技术大学,2018: 1-112.
XuB. Research on high efficiency femtosecond laser integration of functional microfluidic chips[D]. Hefei: University of Science and Technology of China, 2018: 1-112.
[28] Wang X D, Yu H B, Yang T, et al. Density regulation and localization of cell clusters by self-assembled femtosecond-laser-fabricated micropillar arrays[J]. ACS Applied Materials & Interfaces, 2021, 13(49): 58261-58269.
[29] Vrancken N, Ghosh T, Anand U, et al. Nanoscale elastocapillary effect induced by thin-liquid-film instability[J]. The Journal of Physical Chemistry Letters, 2020, 11(7): 2751-2758.
[30] 孙玉金. 液滴/气泡与微结构表面的粘附机制研究[D]. 徐州:中国矿业大学,2018: 109-111.
SunY J. Study of adhesion mechanism between water droplet/bubbles and patterned surfaces[D]. Xuzhou: China University of Mining and Technology, 2018: 109-111.
[31] Li P W, Yu H B, Wang X D, et al. Self-assembled microcage fabrication for manipulating and selectively capturing microparticles and cells[J]. Optics Express, 2021, 29(7): 11144-11157.
Article Outline
杨婷, 孙丽娜, 代国朋, 吕孝峰, 王晓朵. 基于多光子聚合微笼阵列的单细胞捕获方法[J]. 中国激光, 2022, 49(24): 2407104. Ting Yang, Lina Sun, Guopeng Dai, Xiaofeng Lü, Xiaoduo Wang. Single Cell Capture Method Based on Multiphoton Polymerization Microcage Arrays[J]. Chinese Journal of Lasers, 2022, 49(24): 2407104.