数字全息成像定量监测癌细胞凋亡过程形态变化(特邀)
0 引言
程序性细胞死亡(Programmed Cell Death,PCD)可由不同分子途径的激活引起[1],不同的细胞死亡过程具有不同的形态和生化特征。细胞凋亡是一种基因调控的程序性细胞死亡,通过消除生理冗余、物理损伤和异常细胞来控制多细胞生物和组织的发育[2]。放疗和化疗对于癌症患者术前和术后的治疗是必不可少的。化疗药物主要通过促进癌细胞凋亡来破坏癌细胞并抑制其增殖。抗癌药物的功效是通过它们识别癌细胞和选择性地促进其凋亡的能力来衡量的。抗癌药物敏感性测试(Drug Sensitivity Test,DST)是一种根据敏感性确定治疗肿瘤最有效药物的方法。肿瘤的基因型和发病机制各不相同,肿瘤可能对一种或多种药物产生耐药性,或对多种药物表现出敏感性[3]。因此,检测DST中药物诱导的癌细胞凋亡,对于降低耐药性和提高药敏试验的效率,实现更有效的个性化治疗具有重要意义[4]。目前检测细胞凋亡的方法主要通过检测与细胞凋亡相关的细胞形态和表面标志物的变化。
在整个有机体中,细胞凋亡会导致细胞的受控分解,从而避免任何细胞内介质的释放,但在体外,细胞凋亡会进入继发性坏死的终末期,导致细胞膜完整性的丧失以及随后细胞内容物释放到周围细胞外空间[5-6]。流式细胞术、膜蛋白[7]、TUNEL分析[8]等方法对细胞凋亡判别的特异性较差。而细胞凋亡过程会表现出典型的、明确的形态学变化,包括质膜起泡、染色质浓缩以及核断裂和凋亡小体的形成等[9-10]形态学标准被认为是细胞凋亡的最可靠依据[11]。
数字全息显微(Digital Holographic Microscopy,DHM)是一种干涉成像技术,可以无创地提供与细胞固有特性相关的丰富的细胞内信息。它可以根据折射率(Refractive Index,RI)对比度识别无标记细胞,而无需对细胞进行荧光标记[12],因此在生物医学领域有着广泛的应用,如细胞计数[13]、疾病诊断[14-15]、癌细胞分离[16-17]、治疗评估[18]。细胞的RI是一个固有的光学参数,它描述了光穿过细胞产生的光程差,与细胞的生物物理特性相关。此外,DHM能够提供定量地相位信息,这使得它与大多数仅能收集光强度的传感器有很大不同。而定量相位图像除了能够提供有关形态学的信息外,还能够计算整个细胞内的干重(Dry Mass,DM)[19-21]。因此数字全息显微成像技术被用来对细胞生长过程及生理活动进行动态监测,如细胞分裂[22]、细胞迁移[23]、细胞在外界刺激下的形态变化[24-27]等。
本文采用数字全息显微成像方法对化疗药物诱导的癌细胞凋亡过程中的形态变化进行记录与分析。实验过程中,通过对卵巢癌细胞A2780加入临床治疗中常用的化疗药物顺铂诱导癌细胞凋亡,利用数值方法再现出癌细胞完整的凋亡过程及细胞膜破裂的定量相位图像,进而从中提取细胞形态参数相位均值及细胞干重对癌细胞凋亡过程中的形态变化进行表征。结果表明,生长状态的癌细胞与凋亡期及死亡细胞的相位图像及其形态参数上有极为明显的差异。因此,本文方法能够在无需荧光标记的情况下对癌细胞的凋亡细胞和死细胞进行区分,为个体化治疗中的体外药敏试验确定和选择最有效的化疗药物,并确定其有效剂量提供一种更加经济、方便的检测方法。
1 材料及方法
1.1 细胞培养
本文研究的细胞系为上皮性卵巢癌细胞A2780细胞。细胞培养过程中,A2780细胞生长在1640(RPMI Medium 1640 basic 1X,GIBCO,中国)培养基中,培养基中添加l-谷氨酰胺、15 mmol/L HEPES和10%胎牛血清(GIBCO 10099-141,澳大利亚)。培养环境为5% CO2,温度为37℃。将癌细胞接种于35 mm的Willco玻璃培养皿中,培养24 h后,将化疗药物顺铂(Sigma-Aldrich,Steinheim,德国)加入培养基中,最终培养基中顺铂药物浓度为20 μg/mL。加入药物之后,立即将细胞培养皿置于观测平台上进行细胞成像。
1.2 数字全息显微图像记录
采用离轴式数字全息显微成像系统对细胞进行成像[28],光路结构及光路示意如
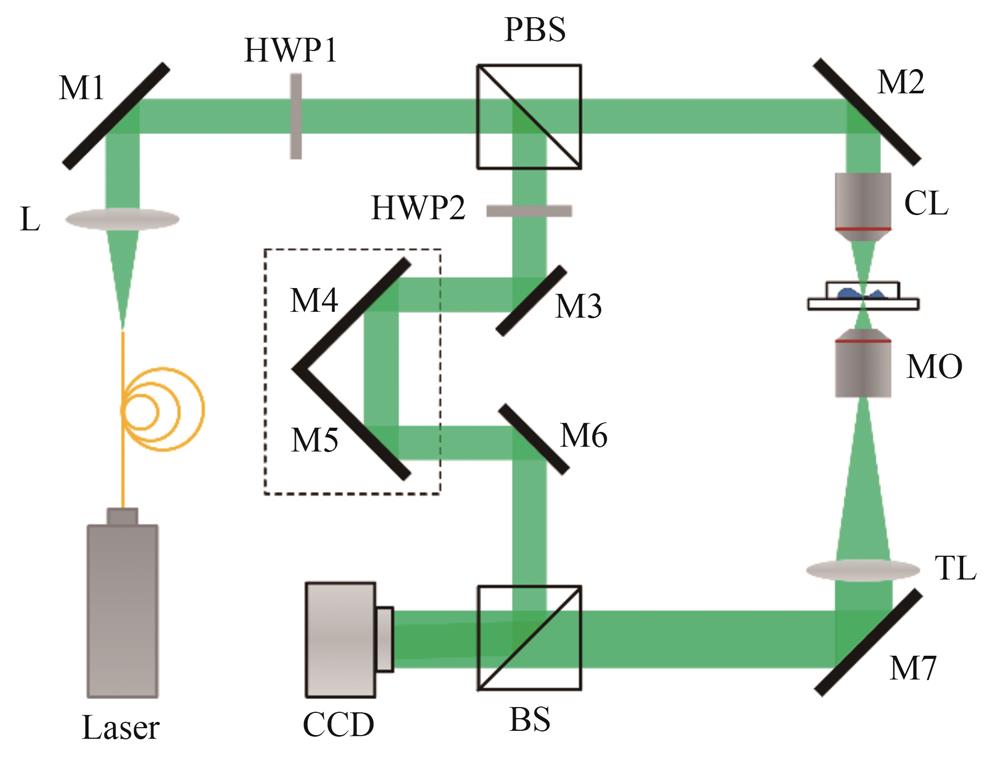
图 1. 离轴数字全息显微成像系统光路结构示意
Fig. 1. Optical path structure of off-axis digital holographic microscopic imaging system
1.3 细胞形态参数计算
1.3.1 细胞相位图像再现处理过程
全息干涉图像由物光波和参考光波叠加形成,全息图的再现过程是利用原参考光
对数字图像形式记录的细胞全息图像,经过数值衍射再现过程能够得到反映细胞折射率信息的相位图像。首先,在进行衍射再现之前,需要对全息图像进行切趾[29]和空间滤波[30]。切趾操作是将全息图像乘以一个二维三次样条插值函数,去除由于CCD的大小有限所产生的衍射条纹对波前的影响。由于离轴记录的全息图像的零级像、+1级像和-1级像相互分离,因此通过将全息图的空间频谱乘以一个带通空间滤波器对全息图像进行滤波即可得到单独的衍射项。然后,采用角谱传播算法对全息图像进行数值传播[31],该方法的优势在于无论传播距离如何,目标图像的大小保持不变。为了消除由于显微物镜的使用、光学元件的缺陷以及实验系统等因素产生的像差对计算细胞相位的影响,采用基于泽尼克多项式模型的数值参数透镜(Numerical Parametric Lenses,NPL)的数值方法来补偿像差[32-33]。此外,数字全息具备进行数值重聚焦的能力,通过对细胞区域计算出最佳的传播距离[34],来补偿图像记录过程中产生的细胞离焦。最后,对细胞相位图像进行解包裹,将约束在-π和π之间的相位值进行解构[35],即可得到数值连续的细胞相位图像。
1.3.2 细胞相位及干重参数计算
为了计算单个细胞在死亡的过程中形态随时间的变化,采用最大类间方差阈值分割方法将单个细胞从背景相位中分割出来。由全息记录重建的细胞相位图像实际上是由于细胞与培养液之间的折射率不同,照明光波在经过不同区域时产生的相位差,即
式中,
式中,
2 实验结果
2.1 癌细胞死亡过程相位图再现结果
实验过程中,将培养皿中贴壁生长的癌细胞在加入药物前置于数字全息显微观测平台上采集一张全息图像,并在加入药物后每1 min采集一张全息图像,实验过程进行9 h,共采集540张全息图。之后将采集到的全息图进行数值重建,得到相位图像,

图 2. A2780细胞加药前与加药9 h后的相位图像
Fig. 2. Phase images of A2780 cells before drug and with drug for 9 h

图 3. 加药后癌细胞调亡过程中形态随时间变化
Fig. 3. Morphological changes of cancer cells death after exposing to drug
2.2 癌细胞凋亡过程参数变化
为了更加准确地描述癌细胞产生破裂死亡过程中的形态变化,对

图 4. 癌细胞调亡过程中相位均值随时间的变化
Fig. 4. Changes of mean phase shift with time in the process of cancer cell death
对细胞内干重的计算结果如

图 5. 癌细胞调亡过程中细胞干重随时间的变化
Fig. 5. Changes of cell dry mass with time in the process of cancer cell death
3 结论
本文采用数字全息显微系统对无任何化学标记的癌细胞加入药物后的细胞形态变化过程进行了记录。通过对记录的全息干涉图像进行切趾、频域滤波、角谱传播、像差补偿及数值自动聚焦等过程完成了对细胞相位图像的数值再现。为了对癌细胞在化疗药物下凋亡过程中细胞形态变化进行分析,从相位图像中分割出单个细胞相位图像,提取其相位均值及细胞干重并绘制出其参数变化曲线。结果表明,癌细胞在凋亡至裂解前,细胞干重没有较明显的变化,而在细胞膜裂解瞬间,细胞内物质流出,细胞干重急剧减小。而细胞相位均值在裂解前不断增大,而细胞干重并未明显增大表明细胞出现了收缩,这与已有研究中的细胞形态变化的结论一致。因此,数字全息显微技术提供的定量相位图像能够反映癌细胞凋亡的特异性形态变化,能够在无需荧光标记的情况下对癌细胞的凋亡细胞和死细胞进行区分,对体外抗癌药物的筛选及个性化治疗具有重要意义。
[1] KRYSKO D V, BERGHE T V, D'HERDE K, et al. Apoptosis and necrosis: detection, discrimination and phagocytosis[J]. Methods, 2008, 44(3): 205-221.
[2] TARAPHDAR A K, ROY M, BTTACHARYA R K. Natural products as inducers of apoptosis: implication for cancer therapy and prevention[J]. Current Science, 2001: 1387-1396.
[3] BALYAN J, KRIZOVA A, GUNULEC J, et al. Multimodal holographic microscopy: distinction between apoptosis and oncosis[J]. PloS one, 2015, 10(3): e0121674.
[4] LIU Kuan, LIU Pengcheng, LIU Run, et al. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry[J]. Medical Science Monitor Basic Research, 2015, 21: 15.
[5] WOLDERS F, ANDERSSON H, VAN DEN BERG A, et al. Apoptosis induced kinetic changes in autofluorescence of cultured HL60 cells-possible application for single cell analysis on chip[J]. Apoptosis, 2004, 9(6): 749-755.
[6] DUPREZ L, WIRAWAN E, BERGHE T V, et al. Major cell death pathways at a glance[J]. Microbes and Infection, 2009, 11(13): 1050-1062.
[7] KRYSKO, DE RIDDER L, CORNELISSEN M. Phosphatidylserine exposure during early primary necrosis (oncosis) in JB6 cells as evidenced by immunogold labeling technique[J]. Apoptosis, 2004, 9(4): 495-500.
[8] FREUDE B, MASTERS T N, KOSTIN S, et al. Cardiomyocyte apoptosis in acute and chronic conditions[J]. Basic Research in Cardiology, 1998, 93(2): 85-89.
[9] SHI Yigong. Mechanisms of caspase activation and inhibition during apoptosis[J]. Molecular Cell, 2002, 9(3): 459-470.
[10] MOUJALLED D, STRASSER A, LIDDERL J R. Molecular mechanisms of cell death in neurological diseases[J]. Cell Death & Differentiation, 2021, 28(7): 2029-2044.
[11] MAHDI E J, ALSHAHRANI A M, ABDULSATAR A A, et al. Morphological evaluation of apoptosis induced by salicylates in HT-1080 human fibrosarcoma cells[J]. Journal of Microscopy and Ultrastructure, 2014, 2(1): 20-27.
[12] SEO S, SU T W, TSENG D K, et al. Lensfree holographic imaging for on-chip cytometry and diagnostics[J]. Lab on a Chip, 2009, 9(6): 777-787.
[13] RA H K, KIM H, YOON H J, et al. A robust cell counting approach based on a normalized 2D cross-correlation scheme for in-line holographic images[J]. Lab on a Chip, 2013, 13(17): 3398-3409.
[14] UGELE M, WENIGER M, LEIDENDERGER M, et al. Label-free, high-throughput detection of P. falciparum infection in sphered erythrocytes with digital holographic microscopy[J]. Lab on a Chip, 2018, 18(12): 1704-1712.
[15] LENZ P, BETTENWORTH D, KRAUSEWITZ P, et al. Digital holographic microscopy quantifies the degree of inflammation in experimental colitis[J]. Integrative Biology, 2013, 5(3): 624-630.
[16] ROITSHTAIN D, WOLBROMSKY L, BAL E, et al. Quantitative phase microscopy spatial signatures of cancer cells[J]. Cytometry Part A, 2017, 91(5): 482-493.
[17] RUBIN M, STEIN O, TURKO N A, et al. TOP-GAN: Stain-free cancer cell classification using deep learning with a small training set[J]. Medical Image Analysis, 2019, 57: 176-185.
[18] BELASHOV V, ZHIKHOREVA A A, BELYAEVA T N, et al. Digital holographic microscopy in label-free analysis of cultured cells' response to photodynamic treatment[J]. Optics Letters, 2016, 41(21): 5035-5038.
[19] BARER R, TKACZYK S. Refractive index of concentrated protein solutions[J]. Nature, 1954, 173(4409): 821-822.
[20] POPESCU G, PARK Y K, LUE N, et al. Optical imaging of cell mass and growth dynamics[J]. American Journal of Physiology-Cell Physiology, 2008, 295(2): C538-C544.
[21] GHENIM L, ALLIER C, OBEID P, et al. A new ultradian rhythm in mammalian cell dry mass observed by holography[J]. Scientific Reports, 2021, 11(1): 1-11.
[22] ZLOTEK-ZLOTKIEWICZ E, MONNIER S, CAPPELLO G, et al. Optical volume and mass measurements show that mammalian cells swell during mitosis[J]. Journal of Cell Biology, 2015, 211(4): 765-774.
[23] JANICKE B, KARSNAS A, EGELBERG P, et al. Label‐free high temporal resolution assessment of cell proliferation using digital holographic microscopy[J]. Cytometry Part A, 2017, 91(5): 460-469.
[24] LEE Y H, LEE C C, HUANG C H, et al. Laminar shear stress promotes nicotine-induced inflammation and hemostatic expression in human endothelial cells[J]. Cellular and Molecular Bioengineering, 2016, 9(3): 466-477.
[25] FEUTH M, VICAR T, GUMULEC J, et al. Quantitative phase dynamics of cancer cell populations affected by blue light[J]. Applied Sciences, 2020, 10(7): 2597.
[26] CAO Runyu, XIAO Wen, WU Xintong, et al. Quantitative observations on cytoskeleton changes of osteocytes at different cell parts using digital holographic microscopy[J]. Biomedical Optics Express, 2018, 9(1): 72-85.
[27] CAO Runyu, XIAO Wen, PAN Feng, et al. Displacement and strain mapping for osteocytes under fluid shear stress using digital holographic microscopy and digital image correlation[J]. Biomedical Optics Express, 2021, 12(4): 1922-1933.
[28] MARQUET P, RAPPAZ B, MAGISTRETTI P J, et al. Digital holographic microscopy: a noninvasive contrast imaging technique allowing quantitative visualization of living cells with subwavelength axial accuracy[J]. Optics Letters, 2005, 30(5): 468-470.
[29] CUCHE E, MARQUET P, DEPEURSINGE C. Aperture apodization using cubic spline interpolation: application in digital holographic microscopy[J]. Optics Communications, 2000, 182(1-3): 59-69.
[30] CUCHE E, MARQUET P, DEPEURSINGE C. Spatial filtering for zero-order and twin-image elimination in digital off-axis holography[J]. Applied Optics, 2000, 39(23): 4070-4075.
[31] DE NICHLA S, FINIZIO A, PIERATTINI G, et al. Angular spectrum method with correction of anamorphism for numerical reconstruction of digital holograms on tilted planes[J]. Optics Express, 2005, 13(24): 9935-9940.
[32] COLOMB T, MONTFORT F, KUHN J, et al. Numerical parametric lens for shifting, magnification, and complete aberration compensation in digital holographic microscopy[J]. Journal of the Optical Society of American A, 2006, 23(12): 3177-3190.
[33] XIAO Wen, XIN Lu, CAO Runyu, et al. Sensing morphogenesis of bone cells under microfluidic shear stress by holographic microscopy and automatic aberration compensation with deep learning[J]. Lab on a Chip, 2021, 21(7): 1385-1394.
[34] MEMMOLO P, DISTANTE C, PATURZO M, et al. Automatic focusing in digital holography and its application to stretched holograms[J]. Optics Letters, 2011, 36(10): 1945-1947.
[35] GHIGLIA D C, ROMERO L A. Robust two-dimensional weighted and unweighted phase unwrapping that uses fast transforms and iterative methods[J]. Journal of the Optical Society of America A, 1994, 11(1): 107-117.
Article Outline
辛露, 肖文, 刘雅坤, 张焕芝, 李小平, 潘锋. 数字全息成像定量监测癌细胞凋亡过程形态变化(特邀)[J]. 光子学报, 2022, 51(10): 1017001. Lu XIN, Wen XIAO, Yakun LIU, Huanzhi ZHANG, Xiaoping LI, Feng PAN. Quantitative Monitoring of Morphological Change of Cancer Cells Apoptosis by Digital Holographic Microscopy(Invited)[J]. ACTA PHOTONICA SINICA, 2022, 51(10): 1017001.



